Summary
Antidepressant treatment response is dependent upon numerous individual factors, such as gender, age, disease severity, symptom type, comorbidities, and genetic factors. New advances in pharmacogenetics, neurosurgical intervention, and medications may hold the key to improving treatment response in patients with refractory depression.
- Mood Disorders
- Psychopharmacology
Antidepressant treatment response is dependent upon numerous individual factors, such as gender, age, disease severity, symptom type, comorbidities, and genetic factors. New advances in pharmacogenetics, neurosurgical intervention, and medications may hold the key to improving treatment response in patients with refractory depression. These new advances were the topic of several sessions at the recent American Psychiatric Association Annual Meeting in New Orleans, LA.
Genetics and Treatment Response
Katharina Domschke, MD, PhD, University of Muenster, Muenster, Germany, discussed the role of pharmacogenetics, candidate genes, and imaging genetics in disease progression and therapeutic response. Many of these candidate gene culprits of depression involve the serotonin/noradrenaline/dopamine system. Specifically, the serotonin transporter gene promoter polymorphism (5-HTTLPR), variations in the serotonin 2A receptor (HTR2A), and neuropeptide Y (NPY) have been linked to antidepressant response in patients with mood disorders [Serretti A et al. Mol Psychiatry 2007; Kato M et al. Mol Psychiatry 2010; Dannlowski U et al. Neuropsychopharmacology 2008; Pae CU et al. Psychiatry Res 2010; Domschke K et al. Eur Neuropsychopharmacol 2010].
In a meta-analysis by Seretti and colleagues, 5-HTTLPR was associated with selective serotonin reuptake inhibitor efficacy in patients with depression. The s/s variant of 5-HTTLPR was significantly associated with remission rate (p<0.0001) and response rate (p=0.0002). Similar response rates were observed that were related to the s/l variant (p=0.0002) [Serretti A et al. Mol Psychiatry 2007]. In a more recent meta-analysis by Serretti and colleagues, pooled data from nine major depressive disorder (MDD) studies (a total of 2642 subjects) demonstrated a significant risk modulation (p=0.0005) that was associated with 5-HTTLPR, and pooled data from seven MDD studies that consisted of 801 subjects demonstrated a significant risk modulation that was associated with polymorphisms in the HTR2A gene (p=0.0006). These associations were found to be considerably more robust when accounting for SSRI-induced side effects alone (p=0.0001 for 5-HTTLPR; p<0.0001 for HTR2A) [Serretti A et al. Mol Psychiatry 2010]. The increased risk that was associated with 5-HTTLPR may be owing to altered limbic neural activity, as 5-HTTLPR polymorphisms are thought to influence amygdala activity during conscious emotion processing and may contribute to the risk of disease chronicity [Dannlowski U et al. Neuropsychopharmacology 2008].
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study also found evidence of an association between HTR2A and treatment outcomes in patients with depressive disorders [McMahon FJ et al. Am J Hum Genet 2006]. In this study, 1953 patients with MDD were treated with citalopram, an antidepressant that downregulates HTR2A. An 18% reduction in absolute risk of having no response to treatment was found in patients who were homozygous for the A allele versus those who were homozygous for the other allele. The Munich Antidepressant Response Signature (MARS) study also indicated that HTR2A plays a role in antidepressant treatment response [Horstmann S et al. Neuropsychopharmacology 2010]. However, the STAR*D findings were not replicated in this study due to disparities in study design and sample population.
There is some evidence that suggests that NPY plays a role in antidepressant treatment response, particularly in relation to the treatment of anxious depression. In a recent study by Domschke and colleagues that investigated 5 FPY gene polymorphisms and antidepressant treatment response, the NPY rs16147–399C allele conferred a slow treatment response after 2 weeks (p=0.14) and failure to achieve remission after 4 weeks on antidepressant therapy (p=0.04). This allele was also associated with stronger bilateral amygdala activation and altered emotional processing, as determined by imaging measurements [Domschke K et al. Eur Neuropsychopharmacol 2010].
Catechol-O-methyltransferase (COMT) may also contribute to treatment response and the pathogenesis of mood disorders. The COMT val158met polymorphism has been associated with increased risk for panic disorder, particularly among Caucasian females. Additionally, the risk of worse response after 4 to 6 weeks of antidepressant therapy in patients with MDD was higher in COMT val158met carriers, possibly conferred by decreased dopamine availability. Therefore, individualized antidepressant therapy with adjunct treatment to compensate for a reduction in dopamine availability according to the COMT val158met genotype status may be a beneficial approach to MDD treatment in the future. Further studies are needed before this benefit can be established [Domschke K et al. Am J Med Genet B Neuropsychiatr Genet 2007; Baune BT et al. Neuropsychopharmacology 2008].
Neuromodulatory Interventions
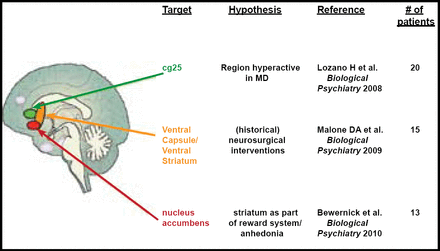
Thomas E. Schlaepfer, MD, University Hospital Bonn, Bonn, Germany, discussed possible interventional approaches to treatment-resistant depression, specifically deep brain stimulation (DBS). There are three potential areas of focus for DBS in depression that offer promising results. They are the subcallosal cingulate gyrus (SCG), the ventral capsule/ventral striatum (VC/VS), and the nucleus accumbens (NAcc) regions of the brain. (Figure 1)
3 Targets for DBS in Depression.
Reproduced with permission from T. Schlaepfer, MD.
SCG is thought to be actively involved in the pathogenesis of depression. In a study by Lozano and colleagues, 21 patients who underwent DBS for treatment-resistant depression were evaluated before and after the procedure to assess the rate of response (determined by ≥50% reduction on the 17-item Hamilton Rating Scale for Depression [HRSD]) or remission (determined by HRSD of ≤7) post-DBS. Metabolic activity within the brain was also assessed using positron emission tomography. Patients were evaluated at 1, 6, and 12 months post-DBS. There was significant improvement in HRSD score from baseline to 12 months (p<0.001). Thirty-five percent of patients achieved response, and 10% achieved remission at 1 month post-DBS. Sixty percent achieved response, and 35% achieved remission at 6 months. The benefit that was seen at 6 months was generally sustained throughout the first year. Metabolic activity was localized to cortical and limbic circuits that were previously thought to be involved in the pathology of depression. The number of serious adverse events was quite low, and no permanent deficits that were related to adverse events were reported. This study provided support for the use of DBS for the treatment of treatment-resistant depression with regard to relative safety and efficacy. The changes in brain metabolism that were observed in this study may also shed some light on the neuropathology of depression [Lozano Am et al. Biol Psychiatry 2008].
The VC/VS region has historically been targeted for refractory depression with irreversible neurosurgical methods. In a study by Malone and colleagues, patients with chronic, severe, refractory depression who received DBS (as a focused and reversible way of modulation) of the VC/VS demonstrated significant improvement in depressive symptoms (as determined by the HRSD, Montgomery-Asberg Depression Rating Scale [MADRS], and Global Assessment of Function [GAF] scale). Mean HRSD scores declined from a baseline score of 33.1 to 14.3 at last follow-up. MADRS and GAF also improved from baseline over the course of the study (MADRS 34.8 at baseline to 15.17 at last follow-up and GAF 43.4 at baseline to 61.8 at last follow-up). Remission rates, based on MADRS scores, were 20% at 6 months and 40% at last follow-up. These results provide support for the use of DBS within the VC/VS region for the treatment of refractory MDD [Malone DA et al. Biol Psychiatry 2009].
The NAcc is thought to be associated with the processing of reward and positive motivation and pleasurable experience. In fact, according to imaging studies, unmedicated depressed patients demonstrate less bilateral VS response to positive stimuli compared with healthy control subjects [Epstein J et al. Am J Psychiatry 2006]. In a recent study by Bewernick and colleagues, DBS to the NAcc was performed in 10 patients with treatment-resistant depression who had not responded to pharmacotherapy, psychotherapy, or electroconvulsive therapy. DBS to the NAcc resulted in improved HRSD scores at 12 months, with 50% of participants meeting the criteria for responders. The number of hedonic activities also increased post-DBS. Positron emission tomography data revealed decreased metabolism in the subgenual cingulate, amygdala, and prefrontal regions of the brain. While no antianxiety effect was observed during this study, DBS to the NAcc did result in better depression scores and interesting metabolic correlations [Bewernick BH et al. Biol Psychiatry 2010].
As Dr. Schlaepfer pointed out, DBS is a promising technique for the treatment of refractory depression and has demonstrated a 50% reduction of symptoms in several studies, which, in light of the extreme treatment refractoriness of the patients who were studied, was unexpected and astonishing. However, there is some debate as to the most appropriate focal region for DBS.
Psychopharmacology
Venlafaxine may be a viable option for patients with refractory MDD. However, there remains some uncertainty about the optimal dose and a therapeutic range for serum levels. Venlafaxine and its active metabolite, O-desmethyvenlafaxine (ODV), blocks serotonin (5HT) and norepinephrine (NA) reuptake, depending on the dose. Qaiser Javed, MBBS, University Hospital Aintree, Liverpool, UK, discussed the relationship between high-dose venlafaxine XL and serum levels of venlafaxine and ODV.
According to a crosssectional, open-label study that included 50 patients with MDD who were on venlafaxine XL (225–525 mg) for at least 12 weeks, there was a significant correlation between ODV levels and mood (p<0.04) and ODV levels and general feelings (p<0.02). There was also a significant correlation between ODV and venlafaxine levels and patient functioning (p<0.03 for both). Venlafaxine dose was positively correlated with serum levels of venlafaxine and ODV.
Faouzi Alam, MD, St. Catherine's Hospital, Liverpool, UK, discussed the tolerability of high-dose venlafaxine XL that was related to this study. Of the 50 patients who were included in this study, 4% reported severe side effects (sweating and weight gain) and considered treatment discontinuation. Moderate side effects included headache, sweating, constipation, sedation, dizziness, and mood swings. The most common mild side effects were sweating, sexual dysfunction, anxiety, and dry mouth. No significant ECG abnormalities were observed. There did not appear to be a correlation between medication dose and side effect frequency or severity, nor did ODV or venlafaxine levels appear to correlate with side effect frequency or severity. Randomized, double-blind clinical trials are required to establish the safety and efficacy of high-dose venlafaxine XL therapy and the impact of serum levels on treatment response.
Refractory MDD poses a treatment challenge for clinicians. However, there are many promising new strategies that may alleviate the therapeutic burden that is associated with treatment-resistant depression.
- © 2010 MD Conference Express