Summary
Patients with chronic obstructive pulmonary disease (COPD) who were managed in primary care settings achieved improvements in physical functioning that were similar to those that were observed in clinical trials of combination therapy with tiotropium+salmeterol. The findings extend the clinical trial results in a carefully controlled treatment environment to an unselected patient population in a real-life clinical setting.
- nursing
- chronic obstructive pulmonary disease
- pulmonary clinical trials
Patients with chronic obstructive pulmonary disease (COPD) who were managed in primary care settings achieved improvements in physical functioning that were similar to those that were observed in clinical trials of combination therapy with tiotropium+salmeterol.
The findings extend the clinical trial results in a carefully controlled treatment environment to an unselected patient population in a real-life clinical setting, according to a report by Thomas Glaab, MD, and Heike Rau-Berger, Boehringer Ingelheim Pharma, Frankfurt, Germany.
To compare the clinical trial and clinical practice experience, 230 office-based pulmonologists in Germany enrolled 1280 patients with COPD who required treatment with a long-acting bronchodilator. Patients initiated treatment with tiotropium at a dose of 5 μg daily (two 2.5 μg puffs) and returned for a follow-up evaluation after 6 weeks.
At baseline and Week 6, physical function was assessed by the 10-item Physical Function (PF-10) Subdomain of the Short Form-36 health status questionnaire. Possible total scores on the assessment ranged from 0 to 100. The primary endpoint of the study was therapeutic success at Week 6, defined as a 10-point increase in the PF-10, validated as the minimally important difference for the PF-10.
Principal secondary outcomes were absolute change in PF-10 score, change in Physician's Global Evaluation (PGE) from baseline to Week 6, and patient satisfaction with treatment, assessed by means of a 7-point scale with a range of “very satisfied” to “very dissatisfied.” Investigators also analyzed the results according to patient smoking status.
Men accounted for about 60% of the total patient population, 35% of whom were current smokers and 48% former smokers. Current smokers averaged 39 pack-years, and ex-smokers had a cumulative exposure of 33 pack-years.
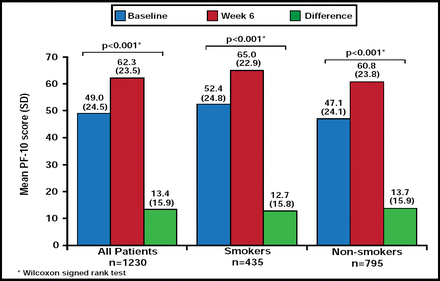
The study population had a mean PF-10 score of 49 at baseline. After 6 weeks of treatment, 61.5% of patients achieved therapeutic success. The rate did not differ between current/former smokers (61.4%) and nonsmokers (61.6%).
The absolute change in PF-10 scores averaged 13.4, representing a statistically significant improvement from baseline (p<0.001; Figure 1). The change in PF-10 averaged 12.7 among smokers (p<0.001) and 13.7 among nonsmokers (p<0.001; Figure 1). The mean absolute change did not differ significantly between smokers and nonsmokers.
Absolute Change in PF-10 Scores.
Reproduced with permission from T. Glaab, MD.
PGE scores reflected a change in patients' general condition during the 6-week treatment period. The proportion of patients who were rated as poor (score of 1 or 2) declined from 16.2% to 3.0%, and the proportion that was rated as good increased from 23% to 54.6%.
With regard to patient satisfaction, 76.9% was satisfied or very satisfied with the inhalation device.
Adverse events were reported by 4% of patients, and 0.5% of patients had serious adverse events. Adverse events led to discontinuation in 2.8% of patients. No patients died during the study.
- © 2010 MD Conference Express