Summary
Bleeding classification systems offer a common language that facilitates comparisons across data sets in multicenter/multinational clinical trials and registries. This article discusses bleeding definitions, the relationship between bleeding and long-term CV outcomes, and the CRUSADE bleeding risk score, among other things.
- myocardial infarction
- coronary artery disease
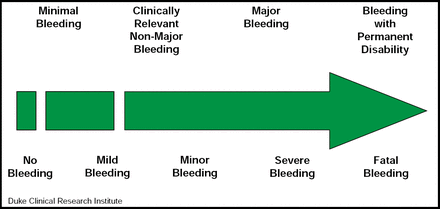
Bleeding classification systems offer a common language that facilitates comparisons across data sets in multicenter/multinational clinical trials and registries. John H. Alexander, MD, Duke University Medical Center, Durham, NC, described commonly used bleeding definitions while evaluating their advantages and limitations. The four major scales that are used in clinical trials today are: TIMI, GUSTO, ISTH, and ACUITY; however, several of the recent major trials have added subdefinitions that were not included in the original scales. In addition, recent large trials have utilized trial-specific definitions (eg, PLATO and CURRENT). Regardless of the scale that is used, however, bleeding should be seen as a continuum (Figure 1). The best bleeding definition depends on the context. Different levels of bleeding may be expected in acute inpatient trials versus long-term trials in stable outpatients, in large phase III trials versus smaller dose-ranging trials, and in data-intensive clinical trials versus large, simple registries. A bleeding definition should be evaluated the same way as a good clinical trial endpoint: it should reflect clinical importance; be sufficiently common in the population to be detected; and be influenced by the intervention that is being studied, easily ascertainable and objectively defined, and standardized across trials/registries.
Bleeding Severity Continuum.
Reproduced with permission from J. Alexander, MD.
“Ultimately, as clinicians, we are interested in the net clinical benefit between ischemic events and bleeding caused by antiplatelet and anticoagulant therapy,” concluded Dr. Alexander. Choosing a primary bleeding definition should be done with this thought in mind.
There appears to be a strong association between bleeding and longer-term adverse cardiovascular outcomes, although a causal relationship between bleeding and mortality remains controversial. John W. Eikelboom, MD, McMaster University, Montreal, Canada, discussed some of the trials that have shown a direct long-term relationship between bleeding severity and adverse outcomes—cardiovascular outcomes, in particular—and identified some challenges for clinicians to better manage bleeding risk. Studies that have helped to define the relationship between bleeding and poor outcome include the OASIS-5 trial, in which >90% of excess deaths occurred in patients with bleeding [Budaj A et al. Eur Heart J 2008]; the OASIS-2 and −4 trials [Eikelboom JW et al. Circulation 2006]; and ACUITY [Mehran R et al. Eur Heart J 2010 (in press)].
This relationship may be explained by several factors, including stopping effective therapies in response to bleeding and the potential effects of stored red blood cells. Koch and colleagues have shown that in patients who are undergoing cardiac surgery, transfusion of red cells that had been stored for more than 2 weeks is associated with a significantly increased risk of postoperative complications, as well as reduced short- and long-term survival [Koch CG et al. N Engl J Med 2008]. These results are consistent with those from other studies [Eikelboom J et al. Am Heart J 2010 (in press)]. It has been suggested that this effect is due, among many other factors, to the depletion of 2,3-DPG in stored red cells [Heaton A et al. Br J Haematol 1989]. Key challenges for clinicians include accurately identifying patients who are at the highest bleeding risk and identifying and implementing strategies that minimize bleeding while still yielding a net benefit for patients. Challenges for researchers include defining and establishing a cause-and-effect relationship between bleeding and subsequent adverse outcomes and controlling for potential confounding, since patients who are more likely to bleed are also more likely to have other adverse events due to the presence of more comorbid conditions.
Richard G. Bach, MD, Washington University School of Medicine, St. Louis, MO, discussed the development and clinical application of the CRUSADE bleeding risk score for patients with non-ST-segment elevation myocardial infarction (NSTEMI) [Subherwal S et al. Circulation 2009]. The CRUSADE score combines eight predictors into a simple validated prediction tool for major bleeding that preserves discrimination across treatment subgroups and complements ischemic risk prediction tools for global risk assessment that enable clinicians to consider all the potential adverse outcomes in patients with NSTEMI prior to the initiation of treatment. The CRUSADE score is limited, in that history of prior bleeding or bleeding diathesis was not collected, and it may underestimate the rate of bleeding in populations of patients who died within 48 hours or in a coronary artery bypass (CABG) population, because bleeding was censored at the time of CABG. Nevertheless, the CRUSADE bleeding score can assist clinicians who are evaluating patients with NSTEMI to quantitatively estimate the risk of major bleeding prior to deciding on therapeutic interventions. A web-based tool to calculate the CRUSADE score is available at www.crusadebleedingscore.org.
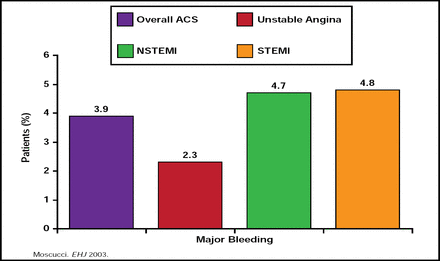
Jean-Pierre L. Bassand, MD, University of Besançon, Besançon, France, noted that there have been dramatic improvements in outcomes of acute coronary syndrome (ACS) treatment over the last 30 years, mostly due to antiplatelet agents, anticoagulants, revascularization/reperfusion/thrombolysis, long-term treatment/secondary prevention, and implementation of guidelines. However, these improvements have been accompanied in many cases by increases in major bleeding (Figure 2). Data from the GRACE Registry indicate that advancing age, female gender, history of bleeding or renal insufficiency, the use of GPIIb/IIIa blockers, and percutaneous interventions (PCI) are some of the factors that are associated with an increased risk of bleeding in patients with NSTEMI (Moscucci M et al. Eur Heart J 2003), as are excess use and incorrect dosing of unfractionated heparin, low-molecular-weight heparin, and GPIIb/IIIa blockers [Alexander KP et al. JAMA 2004], which appear to be even more common in the very patients who are already at risk of bleeding for other reasons [Alexander KP et al. Circulation 2006]. Recently, it has been suggested that bleeding risk may have a genetic component. In particular, CYP2C19*17 carrier status has been shown to be significantly associated with enhanced response to clopidogrel and an increased risk of bleeding [Sibbing D et al. Circulation 2010].
GRACE Registry: Frequency of Major Bleeding in Overall Patient Population.
Reproduced with permission from JP Bassand, MD.
The challenge remains to develop therapies that inhibit platelet activation more effectively without increasing bleeding complications. The inhibition of the platelet protease-activated receptor-1 (PAR-1) for thrombin has been shown to inhibit thrombin-mediated platelet activation without increasing bleeding in preclinical models and small-scale clinical trials [Angiolillo DJ et al. Eur Heart J 2010]. Results from the TRA20P-TIMI 50 [NCT00526474] and TRACER [NCT00527943] trials, which are due in approximately 1 to 2 years, are anxiously awaited.
Deepak L. Bhatt, MD, Boston Healthcare System and Harvard Medical School, Boston, MA, discussed the current strategies that are available to decrease the risk of ACS-associated bleeding. Gastrointestinal (GI) bleeding is the most common form of chronic major bleeding that occurs in patients with ACS. The OCLA and COGENT trials have attempted to determine if prophylactic use of proton pump inhibitors (PPI) reduces the risk of GI bleeding and affects survival outcome. While omeprazole has been shown to significantly decrease inhibition of the platelet P2Y12 receptor by clopidogrel [Gilard M. J Am Coll Cardiol 2008], it is unclear whether this translates to increased rates of clinical adverse ischemic events. Preliminary results from the COGENT trial [Bhatt. TCT Sept 2009] have shown that GI bleeding events are reduced with PPI treatment versus placebo and that survival curves with respect to composite cardiovascular events after 1 year do not appear to differ.
The increasing use of the radial approach to PCI may offer some protection from excess bleeding. Compared with the femoral approach, the use of radial-PCI was associated with a similar rate of procedural success (OR, 1.02; 95% CI, 0.93 to 1.12) but a significantly lower risk for bleeding complications (OR, 0.42; 95% CI, 0.31 to 0.56). The reduction in bleeding complications was more pronounced among patients aged <75 years, women, and patients who were undergoing PCI for ACS [Rao SV et al. J Am Col Cardiol 2008].
In the 1-year results from the HORIZONS-AMI trial, patients with acute STEMI who were undergoing PCI and treated with the thrombin inhibitor bivalirudin had significantly lower rates of protocol-defined major bleeding (5.8% vs 9.2%; HR, 0.61; 95% CI, 0.48 to 0.78; p<0.0001) as well as trial-defined net adverse clinical events (bleeding + ischemic events) than patients who were assigned to heparin plus a GPIIb/IIIa inhibitor (15.6% vs 18.3%; HR, 0.83; 95% CI, 0.71 to 0.97; p=0.022) [Mehran R et al. Lancet 2009]. These results (and similar findings with other recent antithrombotic therapies, such as fondaparinux and dabigatran) offer the hope that future antithrombotic treatment strategies will be able to simultaneously reduce bleeding and ischemic complications, thus resulting in a win-win situation for patients.
- © 2010 MD Conference Express