Summary
Inflammation plays a critical role in all phases of atherosclerosis, and it is triggered in part by lipid entry and retention and subsequent oxidation in the vessel walls. Most of the factors that contribute to plaque instability are related to lipid accumulation and subsequent inflammatory pathway activation. Changing the phenotype of the atherosclerotic lesions could increase plaque stability and reduce clinical events. This article discusses several interventions to reduce inflammation, as well as selected studies regarding the reduction of risk factors.
- inflammatory disease
- lipid disorders
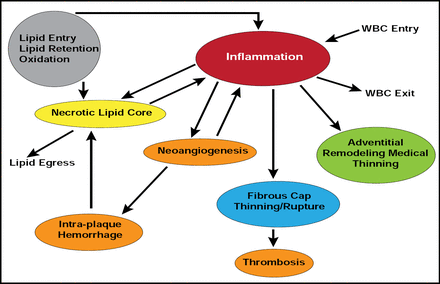
Inflammation plays a critical role in all phases of atherosclerosis, and it is triggered in part by lipid entry and retention and subsequent oxidation in the vessel walls (Figure 1). However, while lipids are essential, they are not sufficient to stimulate atherosclerosis. Lipids induce inflammation, in part, by activating the innate immune signaling pathway, involving toll-like receptor (TLR) 4 and myeloid differentiation primary response gene 88 (MyD88) [Michelson KS et al. Proc Nat Acad Sci 2004].
Inflammation: A Key Determinant of Plaque Development and Progression/Stability.
Reproduced with permission from PK Shah, MD.
Most of the factors that contribute to plaque instability (eg, increased necrotic lipid core, reduced collagen content, increased cap-inflammation, etc.) are related to lipid accumulation and subsequent inflammatory pathway activation. Changing the phenotype of the atherosclerotic lesions could increase plaque stability and reduce clinical events. Prediman K. Shah, MD, Cedars Sinai Heart Institute, Los Angeles, CA, suggested several interventions to alter the phenotype and thus reduce inflammation: low-density lipoprotein (LDL) lowering, increasing high-density lipoprotein (HDL)-based interventions, and anti-inflammatory and immunomodulatory interventions.
Carotid plaque in patients with symptomatic carotid artery stenosis who received 40 mg/day pravastatin (n=11) versus no lipid-lowering therapy (n=13) for 3 months before scheduled carotid endarterectomy displayed decreased lipids, lipid oxidation, inflammation, metalloproteinase activity, and cell death and increased tissue inhibitor of metalloproteinase 1 and collagen [Crisby M et al. Circulation 2001]. These data show that statins can change human plaque phenotype in a favorable way.
Having low HDL is also proinflammatory. Compared with normal individuals, individuals with familial hypoalphalipoproteinemia have increased levels of inflammation, as evidenced by higher hs-CRP levels, particularly if they also have coronary artery disease [Sampietro T et al. Circulation 2002]. Animal studies have shown that plaque lipid content can be dramatically reduced with injections of recombinant HDL (Apo A-I Milano) [Shah PK et al. Circulation 2001] or through gene transfer [Wang L et al. J Am Col Cardiol 2006]. In humans, infusion with human plasma-derived HDL has been shown to reduce plaque lipid and markers of plaque inflammation in plaques that have been removed from lower extremities and improve the cholesterol efflux-promoting capacity of serum [Shaw J et al. Circ Res 2008].
The feasibility of immunomodulating therapy for atherosclerosis has been shown in animal models. Immunization with homologous LDL [Ameli S and Shah PK et al. Arterioscler Thromb Vasc Biol 1996; Nilsson J and Shah PK et al. J Am Col Cardiol 1997] or Apo B-100-related peptide sequence [Chyu KY et al. Biochem Biophys Res Commun 2005] has been shown to reduce aortic atherosclerosis. Human studies of Apo B-100-related peptide vaccine are expected to begin in the summer of 2010, pending investigational new drug approval by the United States Food and Drug Administration. Other novel approaches under investigation include inhibition of Lp-PLA2 with darapladib or sPLA2 using varespladib. Both of these compounds are in early human trials.
Poor control of cardiovascular risk factors, especially hyperlipidemia, diabetes mellitus, smoking, and the metabolic profile, alter the Virchow triad (rheology, substrate, and blood), a primary driver of arterial thrombosis. Their effects include worsening vasoconstriction that causes high shear force and increased platelet deposition; increased tissue factor, which leads to thrombin generation; and increased blood coagulability. James H. Chesebro, MD, University of Massachusetts Medical Center, Worcester, MA, provided data from selected studies, showing that the effect of controlling for even a single risk factor can be significant for reducing arterial thrombus formation.
Reductions in low-density LDL-C levels dilate coronary arteries and microvessels and reduce endothelial dysfunction, platelet deposition, thrombin generation, arterial thrombus, and macrophage adhesion. Studies that used a Badimon perfusion chamber have shown that the use of statins to lower LDL-C is associated with a significant (p<0.05) reduction in arterial blood thrombogenicity and arterial thrombus formation. Lowering LDL-C from a median of 140 mg/dL to 105 mg/dL results in a 20% decrease in arterial thrombus formation [Rauch U et al. Atherosclerosis 2000]. These results are similar to those that were achieved by adding clopidogrel to aspirin (23% decrease in arterial thrombus formation; p<0.05) [Helft G et al. Arterioscler Thromb Vasc Biol 2000].
As shown in the ARMYDA-ACS [Patti G et al. J Am Col Cardiol 2007] and NAPLES II [Briguori et al J Am Col Cardiol 2009] trials, acute treatment with statins just prior to percutaneous coronary intervention (PCI) can reduce 30-day major adverse cardiac events. High-dose statin therapy is also associated with a reduction of coronary events, even in patients who are treated only medically (16% MIRACL; 25% A to Z, and 28% PROVE-IT). In the SAGE (Study Assessing Goals in the Elderly) study, which comprised older subjects (aged 65 to 85 years) with acute coronary syndromes, intensive statin therapy was associated with a significant reduction in all-cause death (HR, 0.33; 95% CI, 0.13 to 0.83; p=0.014) compared with moderate therapy, in addition to the reductions in ischemia that were observed with both therapies [Deedwania P et al. Circulation 2007].
Controlling diabetes is also an important factor in decreasing thrombotic events. The CD-40 ligand is a mediator and risk marker for inflammation and thrombosis that is increased in individuals with diabetes. The use of insulin-sensitizing thiazolidinediones for controlling diabetes results in a decrease in CD-40 plasma levels and a 29% decrease in thrombotic events [Varo N et al. Circulation 2003].
As shown by the results of the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study, intensive diabetes therapy has long-term beneficial effects on the risk of cardiovascular disease (CVD) in patients with type 1 diabetes. In this study, in which patients were followed for a mean of 17 years, intensive treatment reduced the risk of any CVD event by 42% (95% CI, 0.09 to 0.63; p=0.02) and the risk of nonfatal myocardial infarction (MI), stroke, or death from CVD by 57% (95% CI, 0.12 to 0.79; p=0.02). The decrease in glycosylated hemoglobin values during the DCCT was significantly associated with most of the positive effects of intensive treatment on the risk of CVD [Nathan DM et al. N Engl J Med 2005].
Many patients aged under 60 years who present with acute MI are smokers. Smoking is associated with vasoconstriction, increases in TF in the arterial wall, and increases arterial (platelet-rich) thrombus formation. Just stopping smoking can be very beneficial in reducing future cardiovascular events.
“Aggressive risk factor reduction reduces the thrombotic factors of the Virchow triad and clinical events,” said Dr. Chesebro.
- © 2010 MD Conference Express