Summary
Advances in pathophysiology, imaging, and therapies have prompted clinicians to reconsider current disease classifications. This article discusses new definitions that are related to cerebrovascular disease.
- cerebrovascular disease
- episodic & paroxysmal disorders
- myocardial infarction
- ischemia
Advances in pathophysiology, imaging, and therapies have prompted clinicians to reconsider current disease classifications. We have seen this modification in the field of cardiology with the development of an updated “Universal Definition” of myocardial infarction (MI), which defines MI as “myocardial cell death due to prolonged ischemia” [Joint ESC/ACCF/ AHA/WHF Task Force. Circulation 2007]. This definition accounts for new MI detection methods, such as troponin and other serum biomarkers and high-sensitivity imaging techniques. It also allows for a diverse spectrum of pathological presentations, such as coagulation necrosis, contraction band necrosis, and selective myocardial cell necrosis. Most importantly, the definition is based on elements that involve core tissue. New definitions that are related to cerebrovascular disease were the topic of discussion during a session at the 2010 International Stroke Conference in San Antonio, TX.
Transient Ischemic Attack
Gregory W. Albers, MD, Stanford Stroke Center, Stanford, CA, discussed issues with the current definition of transient ischemic attack (TIA) and what is needed to clarify TIA in the future. Modern concepts of brain ischemia are not consistent with the time-related (24 hour) definition of TIA, as many events that occur within a 24-hour time period have resulted in infarction, and this duration does not accurately reflect the typical course of TIA [Easton JD et al. Stroke 2009]. This time constraint also creates a dilemma with regard to tPA. While time is of the essence for tPA, the current definition of TIA may potentially delay the initiation of effective stroke therapies.
Dr. Albers proposes a tissue-based definition in order to synchronize cerebrovascular classification with other ischemic conditions and identify brain injury and vascular pathology through the use of readily available neurodiagnostic tests. Additionally, he suggests replacing the term TIA with “cerebral infarction with transient symptoms.”
As of 2009, the American Heart Association/American Stroke Association Scientific Committee endorsed and recommended a new definition of TIA, which classifies TIA as “a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction” [Easton JD et al. Stroke 2009]. There have been some additional concerns regarding this new definition to which the committee has responded and offered recommendations. In order to avoid arbitrary time limits that are associated with stroke or TIA, the committee suggests removing any reference to time frame from the guidelines. However, the committee encourages the collection of symptom duration data for use in epidemiological studies. This new definition requires brain imaging; therefore, incidence rates may vary according to the availability and utilization of diagnostic imaging techniques. To facilitate consistency within stroke and TIA data, the committee recommends MRI as the preferred imaging modality. While it is not always possible to establish that brain injury has occurred, the term “acute neurovascular syndrome” may be appropriate under these circumstances.
Cerebral Infarction
Jeffrey Saver, MD, UCLA Stroke Center, Los Angeles, CA, discussed the issue of new definitions as they apply to cerebral infarction (CI). He reiterated Dr. Albers' call for a tissue-based definition with a departure from the time-based classification and pointed out that CI definitions among distinguished neuropathology textbooks vary greatly and are often contradictory. For instance, some CI definitions require arterial occlusions or an area of coagulation necrosis versus necrosis with no mention of arterial occlusion in others. The operational details within the textbook definitions, such as the volume of necrosis necessary to be deemed CI or clarification of what a well-circumscribed area of necrosis entails, are also imprecise. Incomplete infarction, such as end-organ injury that is found in the vague area between selective neuronal necrosis and pan-necrosis, also complicates CI classification.
Dr. Saver and colleagues proposed, “CI is defined as brain or retinal cell death due to prolonged ischemia” [Saver J et al. Stroke 2008]. This allows for symptomatic as well as silent infarcts and recognizes both gray and white matter infarcts. CI can be determined through a variety of readily available diagnostic tests, including DWI MRI, and can be easily utilized in a clinical practice setting. This new definition will also foster action in the acute care setting, allowing for more rapid reperfusion decision-making. The idea of a “universal definition” for CI that is convergent with approaches that are taken in allied disciplines, such as cardiology, and promotes improved response times and best clinical practices is a sound mission that deserves serious consideration, concluded Dr. Saver.
Stroke
Scott E. Kasner, MD, University of Pennsylvania Medical Center, Philadelphia, PA, elaborated on the concept of the silent infarct and stroke. The definition of stroke has wavered from “cerebral vascular accident” to “permanent injury to the brain or spinal cord of vascular origin (reduced blood flow or bleeding into or around the brain or spinal cord)” [AHA Stroke Council for US Dept of Justice Public Safety Officers Benefits Program 2009]. However, these definitions were based on consensus and were not formally peer-reviewed, nor were they endorsed by the American Heart Association. Therefore, more specific definitions are needed.
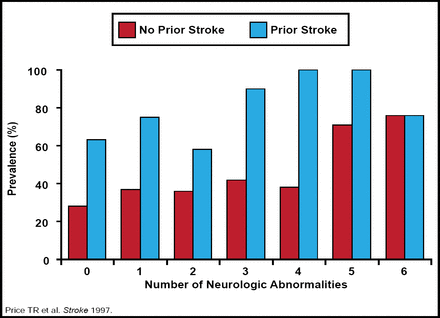
According to pooled data, silent infarcts are quite prevalent and occur in 10% to 28% of stroke study participants [Vermeer et al. Lancet Neurology 2007]. The question remains: are these truly instances of silent infarcts or are many of them cases of inadequate symptom recognition and poor self-reporting? Regardless of the circumstances, it is important to account for this group when reassessing definitions, because the risk of subsequent stroke and dementia is nearly 2-fold in this group (Figure 1) [Price TR et al. Stroke 1997; Reitz et al. Arch Neurol 2009; Tanne & Levine. Arch Neurol 2009]. However, it is unclear how silent infarcts should be categorized in ongoing and future clinical trials and whether or not a silent infarct may be considered a qualifying event. It is also uncertain as to how the MRI reimbursement would operate in the setting of silent infarct.
Silent Infarcts. Are They Really Silent?
Copyright © 1997 American Heart Association. All rights reserved.
Though the definitions of intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) have remained the same, Dr. Kasner voiced concerns about details that are presently being overlooked. For example, should sentinel headache be considered SAH and does SAH always cause permanent injury to the central nervous system? Additionally, subdural and epidural hemorrhage, defined as acute or chronic hemorrhage in the subdural or epidural space, is usually the result of trauma, but occasionally it occurs in patients with coagulopathy or amyloid angiopathy; therefore, it may be appropriately classified as stroke. Further, cerebral venous thrombosis that results in infarction or hemorrhage should likely be qualified as stroke, while headache or intracranial hypertension in the absence of a structural central nervous system injury should be excluded from the stroke definition, said Dr. Kasner. “This is a work in progress right now, but we hope for resolution of this matter by this time next year,” concluded Dr. Kasner.
It is important to structure these definitions so that they are clear and precise and account for advanced diagnostic testing and therapies. Tissue-based, rather than time-based, stratification seems to be a more efficient way of classifying these maladies. Universal definitions will facilitate consistent clinical responses and bridge the gap across specialties.
- © 2010 MD Conference Express