Summary
A subgroup analysis of the Randomized Evaluation of Long-term anticoagulant therapy [RE-LY; NCT00262600] trial revealed that dabigatran 110 mg or 150 mg twice daily is as effective as warfarin for stroke prevention in patients who have had a prior stroke or transient ischemic attack.
- arrhythmias
- neurology clinical trials
- ischemia
- cerebrovascular disease
- episodic & paroxysmal disorders
A subgroup analysis of the Randomized Evaluation of Long-term anticoagulant therapy (RE-LY; NCT00262600) trial revealed that dabigatran 110 mg or 150 mg twice daily is as effective as warfarin for stroke prevention in patients who have had a prior stroke or transient ischemic attack (TIA). Dabigatran is also associated with a lower incidence of any hemorrhage, including hemorrhagic stroke, compared with warfarin.
The RE-LY study was a large, international, multicenter, randomized trial that included 18,113 patients with nonvalvular atrial fibrillation (AF) who were at moderate to high risk of stroke or systemic embolism and had at least one additional risk factor. Patients were randomized to receive dabigatran 110 mg twice daily (1195 had prior stroke and 4819 had no prior stroke), dabigatran 150 mg twice daily (1233 had prior stroke and 4843 had no prior stroke), or warfarin (INR 2.0–3.0; 1195 had prior stroke and 4827 had no prior stroke). The mean observation time was 2 years, and those with renal insufficiency (CrCl <30 ml/min) were excluded from study participation. Events were independently and blindly adjudicated following a PROBE design (prospective randomized open with blinded endpoint evaluation). The primary outcome was stroke or systemic embolism.
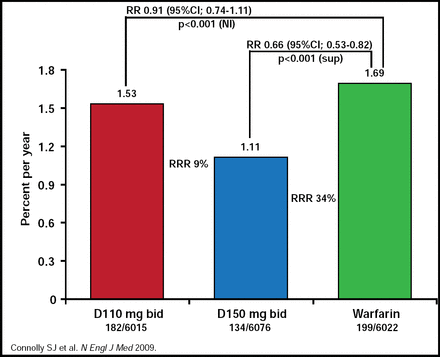
The subgroup analysis includes the secondary stroke prevention part of the RE-LY study that explored the treatment effects of dabigatran versus warfarin in patient population who had a prior stroke or TIA. In the overall RE-LY patients, the rate of the primary outcome was 1.69% per year in the warfarin group, as compared with 1.53% per year in the group that received 110 mg of dabigatran (relative risk with dabigatran, 0.91; 95% CI, 0.74 to 1.11; p<0.001 for noninferiority) and 1.11% per year in the group that received 150 mg of dabigatran (relative risk [RR], 0.66; 0.53 to 0.82; p<0.001 for superiority; Figure 1).
Stroke/SSE All Patients.
Reproduced with permission from H.C. Diener, MD.
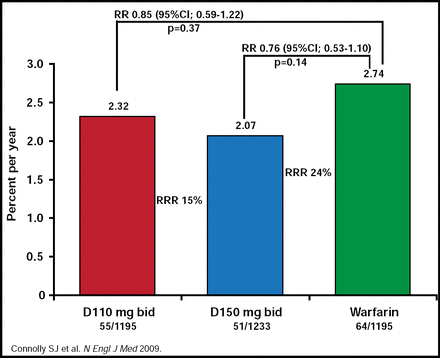
A consistent finding was found in patients with prior stroke or TIA (RR, 0.85; 95% CI, 0.59 to 1.22; p=0.37 for dabigatran 110 mg vs warfarin; RR, 0.76; 95% CI, 0.53 to 1.10; p=0.14 for dabigatran 150 mg vs warfarin; Figure 2).
Stroke/SSE Patients with Prior Stroke or TIA.
Reproduced with permission from H.C. Diener, MD.
Overall, both dabigatran treatments were superior to warfarin with regard to hemorrhagic stroke (p<0.001) Likewise, in the subgroup of patients with prior TIA or stroke, there was an 89% and 73% relative risk reduction in the incidence of hemorrhagic stroke in the dabigatran 110 mg (p=0.003) and dabigatran 150 mg (p=0.009) groups, respectively, compared with warfarin. Intracranial bleeding rates for all patients were lower in the dabigatran groups than in the warfarin group (p<0.001 superior for both dabigatran doses). Intracranial bleeding rates were also lower in patients with prior stroke or TIA compared with warfarin (p<0.001 for dabigatran 110 mg; p=0.007 for dabigatran 150 mg). There was no increase in bleeding rate that was associated with dabigatran and concomitant aspirin use.
The RE-LY study was a large trial that evaluated two dabigatran dose strategies using rigorous adjudication of events. Results of both the main study and the subanalysis are promising. However, there are some potential shortcomings in this study, including the fact that the warfarin arm was not blinded. Results of the subgroup analysis were consistent with the findings of the overall patient cohort; however, the subgroup was too small to demonstrate a statistically significant superiority of the higher dabigatran dose over warfarin, as demonstrated in the overall RE-LY cohort. Further evaluations of the long-term safety and efficacy data from RE-LY data are needed to determine the optimal choice of the dabigatran dose for patients with prior TIA or stroke of treatment with dabigatran.
- © 2010 MD Conference Express