Summary
Cilostazol is significantly more effective than aspirin in preventing recurrent stroke and is associated with fewer hemorrhagic events. This article discusses findings from the Cilostazol Stroke Prevention Study II [CSPS II].
- neurology
- cerebrovascular disease
- ischemia
- neurology clinical trials
Cilostazol (CLZ) is significantly more effective than aspirin in preventing recurrent stroke and is associated with fewer hemorrhagic events. Yukito Shinohara, MD, Federation of National Public Service Personnel Mutual Aid Associations Tachikawa Hospital, Tokyo, Japan, presented findings from the Cilostazol Stroke Prevention Study II (CSPS II).
CSPS II was a multicenter, double-blind, parallel-group, randomized, prospective comparative study that included 2757 patients with CT- or MRI-proven noncardioembolic stroke from 278 Japanese institutes. Efficacy and safety analyses were based on 2672 patients, of whom 1337 were in the CLZ group and 1335 were in the aspirin group. Patients were randomized to receive either CLZ 100 mg twice daily (n=1337) or aspirin 81 mg once daily (n=1335). There was no significant difference in baseline characteristics between the two groups. Treatment duration was 1 to 5 years.
The aim of this study was to establish noninferiority (defined as upper limit of 95% CI for HR ≤1.33) of CLZ compared with aspirin for the prevention of stroke recurrence in patients with noncardioembolic cerebral infarction. The primary endpoint was the occurrence of symptomatic stroke, including recurrence of cerebral infarction, or occurrence of intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH) during the treatment period. Secondary endpoints were the recurrence of symptomatic cerebral infarction, the occurrence of ischemic cerebrovascular events, including cerebral infarction or transient ischemic attack (TIA); death from any cause; and the composite of stroke, TIA, angina pectoris, myocardial infraction (MI), heart failure, or hemorrhage that required hospitalization during the treatment period. Safety endpoints were bleeding events, including ICH, SAH, or hemorrhage that required hospitalization.
The primary endpoint of stroke occurred more frequently in patients who were treated with aspirin (n=119) than with CLZ (n=82) (HR, 0.743; 95% CI, 0.564 to 0.981; p=0.0357). This demonstrated a 25.7% relative risk reduction in stroke occurrence in patients who were treated with CLZ. There was also a 20.1% relative risk reduction in the composite secondary endpoint of stroke, TIA, angina pectoris, MI, heart failure, or hemorrhage that required hospitalization in patients who were treated with CLZ versus aspirin (p=0.0437). Results for the remaining secondary endpoints—cerebral infarction, transient ischemic attack (TIA), or death from any cause—were similar for both groups (Table 1).
Incidence of Primary and Secondary Endpoints.
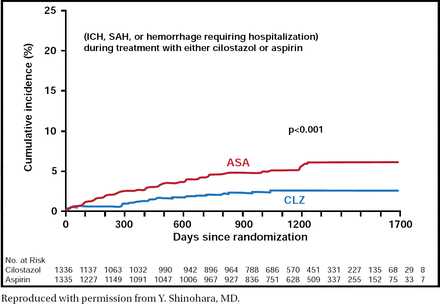
Safety results also demonstrated favorable outcomes with the use of CLZ versus aspirin. Hemorrhagic events occurred less frequently in patients who were treated with CLZ than in those who were treated with aspirin (p<0.001; Figure 1). Adverse drug reactions that resulted in treatment discontinuation occurred in 19.8% of patients in the CLZ group versus 12.2% in the aspirin group. The most common adverse events other than bleeding were headache, diarrhea, palpitations, dizziness, and tachycardia in the CLZ group and hypertension and constipation in the aspirin group.
Safety Endpoint: Hemorrhagic Events.
Reproduced with permission from Y. Shinohara, MD.
This study demonstrated noninferiority of CLZ compared with aspirin in preventing stroke recurrence. In fact, CLZ was significantly more effective and was associated with a lower incidence of bleeding compared with aspirin. Based on these results, Dr. Shinohara concluded that CLZ is a possible treatment option for the prevention of stroke recurrence in patients with noncardioembolic stroke who can tolerate long-term administration of CLZ. Subgroup and cost-effectiveness analyses of this study are ongoing.
- © 2010 MD Conference Express