Summary
A number of promising new antithrombotic agents intended to prevent atherothrombotic events in patients with coronary artery disease (CAD) and atrial fibrillation are currently being investigated. This article discusses the progress that is being made with adenosine diphosphate (ADP) inhibitors, antithrombins and factor Xa inhibitors. Other topics include the the problem of stent thrombosis and possible solutions.
- coronary artery disease
- thrombotic disorders
A number of promising new antithrombotic agents intended to prevent atherothrombotic events in patients with coronary artery disease (CAD) and atrial fibrillation are currently being investigated. Marc S. Sabatine, MD, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, discussed the progress that is being made with adenosine diphosphate (ADP) inhibitors, antithrombins and factor Xa inhibitors.
Clopidogrel is an effective, oral antiplatelet agent (thienopyridine class) that is currently used in patients with acute coronary syndromes (ACS) or those who are undergoing percutaneous coronary intervention (PCI). While trials have shown that clopidogrel is effective in these settings overall, inter-patient variability in response to clopidogrel has recently become a concern. Gurbel and colleagues [Gurbel PA et al. Circulation 2003] were the first to show that there is a marked interindividual variability in clopidogrel's ability to reduce ADP-induced platelet aggregation. Patients with the highest pretreatment platelet reactivity remained the most resistant at 24 hours after treatment (p<0.0001) and the least protected from recurrent cardiovascular (CV) events [Gurbel PA et al. Circulation 2003; Matetzky S et al. Circulation 2004].
This variability has been attributed to metabolic and pharmacogenetic factors. Clopidogrel is a prodrug that must be metabolized via 2 steps – first in the peripheral blood and then in the liver by cytochrome P450 (CYP) before it becomes active. Individual differences in clopidogrel's metabolic pathway have been suggested as a reason for this variability. Genetic factors have also been suggested as a reason for clopidogrel's response variability. Patients who carry CYP2C19 loss-of-function alleles, resulting in altered hepatic metabolism, have a relative reduction of 32.4% in plasma exposure to the active metabolite of clopidogrel, as compared with noncarriers (p<0.001), which can lead to a relative increase of as much as 53% in the composite primary efficacy outcome of the risk of death from CV causes, MI, or stroke (12.1% vs 8.0%; HR for carriers, 1.53; 95% CI, 1.07 to 2.19; p=0.01), and an increase by a factor of 3 in the risk of stent thrombosis (2.6% vs. 0.8%; HR, 3.09; 95% CI, 1.19 to 8.00; p=0.02) [Simon T et al. N Engl J Med 2009; Mega JL et al. N Engl J Med 2009].
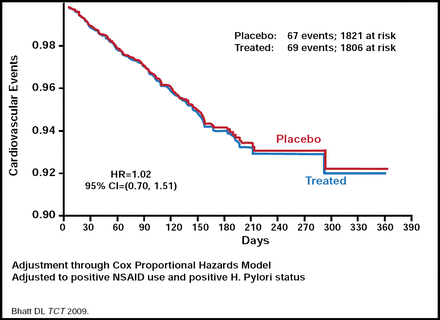
Dr. Sabatine discussed the controversy of drug-drug interactions with clopidogrel use. Some recent studies have suggested that the response to clopidogrel is diminished in patients who received proton pump inhibitor (PPI) treatment [Gilard M et al. J Am Coll Cardiol 2008], which may lead to an increased risk for CV events. The PPI omeprazole inhibits CYP2C19 proteins which are involved in clopidogrel metabolism [Dunn SP et al. AHA 2008]. On the other hand, preliminary results from the COGENT trial, the only randomized controlled trial to compare the PPI omeprazole with placebo in patients who are taking clopidogrel, found absolutely no difference in the risk of CV events or MI, but there was a benefit in terms of reduced GI adverse events in patients who are taking PPIs (Figure 1) [Bhatt DL. TCT 2009]. Dr. Sabatine concluded from these data that it is likely safe to administer clopidogrel to patients who are taking PPIs, but the doses of clopidogrel and the PPI should be given at different times.
COGENT Trial: CV Events.
Like clopidogrel, prasugrel is a prodrug that is metabolized into its active metabolite in the liver. Both clopidogrel and prasugrel are thienopyridines that inhibit the platelet P2Y12 ADP receptor. Inhibition of platelet aggregation (IPA) after prasugrel is significantly higher (p< 0.01), more rapid, and consistent than that after clopidogrel [Brandt J et al. Am Heart J 2007]. The degree of interpatient variability in the percentage of platelet inhibition is much less than with clopidogrel. The TRITON-TIMI 38 study reported that in ACS patients who are planned for PCI, prasugrel therapy, compared with clopidogrel, was associated with a 19% reduction in CV deaths, MI or stroke events (12.1% of patients who received clopidogrel vs 9.9% of patients who received prasugrel; p<0.001). There was a 52% decrease in the incidence of stent thrombosis (2.4% vs 1.1%; p<0.001), but an increased risk of TIMI major bleeding (2.4% vs 1.8%; p=0.03), including fatal bleeding (0.4% vs 0.1%; p=0.002) with prasugrel use [Wiviott SD et al. Am Heart J 2006; Wiviott SD et al. N Engl J Med 2007; Wiviott SD et al. Lancet 2008; Wiviott SD et al. Circulation 2008]. There appeared to be greater benefits for patients without prior stroke/MI, who were younger, and had lower body weight.
Ticagrelor is a nonthienopyridine oral reversible P2Y12 antagonist that does not require metabolic activation. ACS patients, either pretreated or naïve to clopidogrel, exhibit greater mean IPA after ticagrelor compared with clopidogrel [Storey R et al. J Am Coll Cardiol 2007]. In a randomized trial of 18,624 patients who presented across the spectrum of ACS, ticagrelor significantly reduced the rate of the primary composite endpoint of CV death, MI, or stroke compared with clopidogrel (9.8% vs. 11.7%, p=0.0003). There was no significant difference in major bleeding between treatment groups; however, ticagrelor was associated with a higher rate of major bleeding that was not related to coronary-artery bypass grafting (4.5% vs 3.8%, p=0.03) [Wallentin L et al. N Engl J Med 2009].
Recently, factor Xa inhibitors, such as otamixaban (IV), and the oral drugs rivaroxaban and apixaban, have been proposed as possible additions to antithrombotic therapy for patients with ACS and are currently under investigation.
Each of these agents has shown promise in possibly reducing major ischemic events in their Phase II studies; however, dose-dependent increases in bleeding were seen with rivaroxaban and apixiban [Mega JL et al. Lancet 2009; Sabatine MS et al. Lancet 2009; Alexander JH et al. Circulation 2009]. Phase III studies are underway to further evaluate the safety and efficacy of these agents.
Deepak L. Bhatt, MD, VA Boston Healthcare System, Boston, MA, discussed the problem of stent thrombosis and possible solutions. Though the overall rates of death and MI do not differ significantly between the bare metal (BMS) and drug-eluting stents (DES) [Stone G et al. N Engl J Med 2007], DES appear to be associated with a 4- to 5-fold increase in the risk for late thrombosis (around 1 year) compared with BMS [Bavry AA et al. Am J Med 2006; Bavry AA and Bhatt DL. Lancet 2008]. This risk is felt to be attributed primarily to early termination or interruption of dual antiplatelet therapy. Thus, current recommendations specify that DES generally should be utilized only if individuals can continue uninterrupted dual antiplatelet therapy for at least 12 months following stent placement.
The second-generation stents, such as the everolimus- and zotarolimus-eluting stents, appear to be more effective than BMS at reducing stent thrombosis, target vessel failure (TVF), and composite rates of death and MI [Stone G. TCT 2009; Leon M. TCT 2009], but more studies are needed, stated Dr. Bhatt.
The platelet, once thought to be involved solely in clot formation, is now known to be a key mediator in inflammation, thrombosis, and atherosclerosis. Thus, antiplatelet agents have become paramount in the prevention and management of various diseases that involve the CV, cerebrovascular, and peripheral arterial systems. Though atherothrombosis is the leading cause of CV morbidity worldwide, the Reduction of Atherothrombosis for Continued Health (REACH) Registry has shown that it remains largely undertreated and undercontrolled in many regions of the world, [Bhatt DL et al. JAMA 2006] even though readily available aspirin has proven to be effective treatment for preventing mortal thrombotic events. The reasons for underutilization of aspirin may include physicians who fail to prescribe, noncompliance, nonabsorption, and intolerance.
Besides aspirin, clopidogrel, is a widely used antiplatelet agent that is indicated to prevent stent thrombosis and other thrombotic events in patients who are at risk for atherothrombosis. Like aspirin, resistance to and variability in platelet responsiveness to clopidogrel are common and serious problems, which may lead to either an increased risk for thrombotic events due to non-/low responsiveness or an increased risk for bleeding due to greater sensitivity [Gurbel PA et al. Circulation 2003]. One approach to offset clopidogrel nonresponsiveness (NR) is to increase the dose. A 600-mg clopidogrel loading dose significantly (p<0.001) reduces the incidence of NR (8%) and high posttreatment platelet aggregation compared with a 300-mg dose (NR=28% to 32%) [Gurbel PA et al. J Am Coll Cardiol 2005].
According to the Clopidogrel REsistance and Stent Thrombosis (CREST) Study higher posttreatment platelet reactivity and non-responsiveness to clopidogrel may be predictive of DES thrombosis [Gurbel PA et al. J Am Coll Cardiol 2005]. The incidence of stent thrombosis is 8.6% in nonresponders and 2.3% in responders (p<0.001) after a loading dose of 600 mg of clopidogrel. Cardiac related death was also significantly higher (p<0.001) in non-responders. [Buonamici P et al. J Am Coll Cardiol 2007].
Prasugrel is more potent than standard-dose clopidogrel in patients with stable CAD. TPRINCIPLE-TIMI 44 study showed that among patients undergoing cardiac catheterization with planned PCI, a loading dose of 60 mg prasugrel resulted in greater IPA than 600-mg clopidogrel (74.8% vs 31.8% IPA). Maintenance therapy with prasugrel 10 mg/day resulted in a greater antiplatelet effect than 150 mg/day clopidogrel (45.4% vs 61.9% IPA) [Wiviott SD et al. Circulation 2007].
“Stent thrombosis is a serious problem,” concluded Dr. Bhatt. These more potent antiplatelet agents are of great clinical interest, provided the reduction in stent thrombosis can be obtained without a major increase in bleeding.
- © 2010 MD Conference Express