Summary
In atrial fibrillation (AF), rhythm control involves a strategy that is designed to restore and maintain sinus rhythm by preventing recurrences, initially by cardioversion (pharmacological or electrical). Rate control focuses on strategies to slow the ventricular response in patients with AF. With either strategy, anticoagulation is also recommended in patients who are at moderate to high risk of stroke.
- Cerebrovascular Disease
- Arrhythmias
In atrial fibrillation (AF), rhythm control involves a strategy that is designed to restore and maintain sinus rhythm by preventing recurrences, initially by cardioversion (pharmacological or electrical). Rate control focuses on strategies to slow the ventricular response in patients with AF. With either strategy, anticoagulation is also recommended in patients who are at moderate to high risk of stroke.
Rate Control Versus Rhythm Control
AF treatment typically begins with the selection of a rhythm control or rate control strategy. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial was designed to compare rhythm control and rate control strategies in 4060 patients with AF and a high risk of stroke or death. Patients who were assigned to rhythm control therapy had a higher risk of 5-year mortality compared with those who were assigned to rate control therapy (23.8% vs 21.3%; p=0.08). Patients who were assigned to rhythm control also had a higher risk of hospitalization (80.1% vs 73.0%; p<0.001) and were more likely to experience adverse drug effects than those who were managed with rate control therapy. Most strokes occurred after warfarin therapy had been discontinued or when the INR was subtherapeutic regardless of the strategy, underscoring the importance of sustained therapeutic anticoagulation [Wyse DG et al. N Engl J Med 2002].
Results from the landmark AFFIRM trial influenced the management of patients with AF who are at high risk of stroke in two important ways, said Albert L. Waldo, MD, Case Western Reserve University, Cleveland, OH. First, AFFIRM demonstrated that pharmacological rate control plus anticoagulation is a valid first-line therapy for patients with persistent or recurrent AF. Second, results from AFFIRM suggested that patients with AF and risk factors for stroke should receive anticoagulation indefinitely, whether they are being managed with pharmacological rate or rhythm control.
Restoration and maintenance of sinus rhythm is a desirable goal in AF patients, because the prevention of recurrences can improve cardiac function and relieve symptoms. Several conditions favor rhythm over rate control and should be considered when individualizing therapy [Fuster V et al. J Am Coll Cardiol 2006]. Patients with substantial symptoms despite effective rate control, younger patients who potentially face decades of AF, and those with conditions that predispose them to left ventricular (LV) diastolic dysfunction may benefit more from initial rhythm control rather than rate control therapy. Physicians should select an antiarrhythmic agent that is least likely to cause harm, based on each patient's specific history and presentation, as initial pharmacological therapy for AF, said Eric N. Prystowsky, MD, St. Vincent Hospital, Indianapolis, IN.
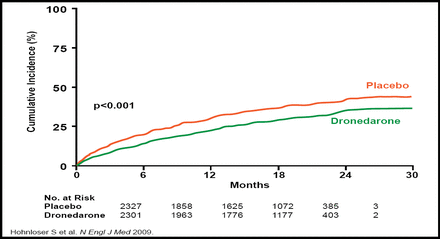
The newest option for rhythm control is dronedarone. Dronedarone is structurally related to amiodarone but does not contain iodine and therefore avoids the iodine-related organ toxicity that is associated with amiodarone. The ATHENA trial evaluated the efficacy of dronedarone in 4628 patients with AF who had additional risk factors for death. Dronedarone significantly decreased the risk of cardiovascular hospitalizations or death from any cause by 24% relative to placebo (p<0.001), meeting the primary study endpoint (Figure 1). In particular, dronedarone reduced hospitalizations that were related to AF by 37% (p<0.001) [Hohnloser SH et al. N Engl J Med 2009].
ATHENA Trial. Primary Outcome.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Dronedarone is not appropriate for all patients with AF, particularly those with heart failure (HF). Indeed, dronedarone is contraindicated in patients with New York Heart Association (NYHA) Class IV HF or NYHA Class II-III HF with a recent decompensation that requires hospitalization or referral to a specialized HF clinic. This contraindication resulted from the ANDROMEDA trial, which was designed to assess the safety of dronedarone in patients with moderate or severe systolic HF and a recent hospitalization. After a median follow-up of 2 months, the study was terminated early due to an excess rate of mortality in the dronedarone group compared with the placebo group (8.1% vs 3.8%), primarily related to worsening HF. The primary composite endpoint of death from any cause or hospitalization for HF did not differ significantly between the dronedarone and placebo groups (17.1% vs 12.6%; p=0.21) [Køber L et al. N Engl J Med 2008].
Management of Stroke Risk in Atrial Fibrillation
Vitamin K antagonists (such as warfarin) are the treatment of choice for the prevention of stroke in high-risk patients with AF. However, due to bleeding risk, patient preference, or an inability to maintain warfarin within the therapeutic range, fewer than 50% of high-risk patients are suitable candidates for oral anticoagulation therapy. The ACTIVE clinical trial program examined several treatment options for reducing vascular events in patients with AF who were at risk for stroke. The three ACTIVE trials were designed to show the noninferiority of clopidogrel plus aspirin to oral anticoagulation with warfarin (ACTIVE W), to compare the effects of dual antiplatelet therapy with clopidogrel plus aspirin versus aspirin monotherapy (ACTIVE A), and to compare the effect of irbesartan versus placebo in addition to the regimens in ACTIVE W and ACTIVE A (ACTIVE I).
In ACTIVE W, oral anticoagulation with warfarin reduced the risk of stroke, systemic embolism, MI, and vascular death compared with dual antiplatelet therapy with clopidogrel and aspirin (p=0.0003) [ACTIVE Investigators. Lancet 2006]. Importantly, warfarin provided this protection against vascular outcomes without raising the risk of major bleeding compared with clopidogrel and aspirin. Among patients with poor INR control (<65% of time in the therapeutic range [TTR]), there was no benefit to warfarin therapy compared with aspirin and clopidogrel with regard to the composite primary endpoint (p=0.61) or to the prevention of stroke, in particular (p=0.42). In contrast, patients with good INR control (TTR ≥65%) had significant benefits with warfarin therapy compared with dual antiplatelet therapy in terms of both the primary endpoint (RR, 2.14; p<0.0001) and protection from stroke (RR, 2.25; p=0.0003). Investigators showed an increasing benefit of oral anticoagulation relative to dual antiplatelet therapy with increasing TTR, beginning at TTR values ≥58%. At TTR values <58%, there was no net benefit from oral anticoagulation [Connolly SJ et al. Circulation 2008].
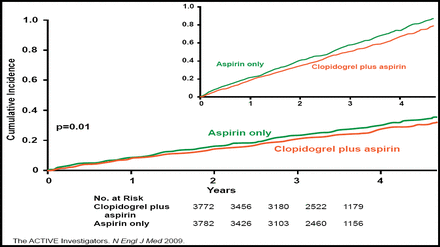
In the ACTIVE A trial, dual antiplatelet therapy with clopidogrel and aspirin reduced the risk of major vascular events compared with aspirin alone for high-risk AF patients who were ineligible for warfarin therapy. ACTIVE A included 7554 patients with documented AF, at least one risk factor for stroke, and no major risk factors for bleeding. All patients were treated with aspirin 75 to 100 mg/day and were randomly assigned to additional treatment with clopidogrel 75 mg/day or placebo. The primary outcome was a composite of major vascular events, including stroke, MI, non-central nervous system (CNS) systemic embolism, and vascular death. After a median follow-up of 3.6 years, dual antiplatelet therapy reduced the risk of the primary outcome by 11% compared with aspirin alone, from an annual rate of 7.6% to 6.8% (HR, 0.89; 95% CI, 0.81 to 0.98; p=0.01; Figure 2). The benefit of clopidogrel was mainly in the reduction of stroke risk, from 3.3% per year with aspirin alone to 2.4% per year with clopidogrel and aspirin (HR, 0.72; 95% CI, 0.62 to 0.83; p<0.001). A safety analysis showed that clopidogrel increased the risk of bleeding in patients who were on long-term aspirin therapy. Compared with patients who were taking aspirin alone, those who were taking clopidogrel and aspirin had a higher rate of major bleeding (1.3% vs 2.0% per year; RR, 1.57; 95% CI, 1.29 to 1.92; p<0.001), including severe bleeding (p<0.001), with a trend toward increased fatal bleeding (p=0.07) [Connolly SJ et al. N Engl J Med 2009].
ACTIVE A Primary Outcome.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Emerging Therapies in Atrial Fibrillation
Current medical antiarrhythmic therapies for AF are palliative rather than curative and therefore have poor long-term efficacy. Adverse effects that are associated with AF therapy, particularly end-organ toxicity, often require early treatment discontinuation, therefore, there is an urgent need for new therapies to control AF. Vernakalant is an atrial selective antiarrhythmic agent with rapid unbinding sodium channel-blocking action and shows with promising efficacy for AF to sinus rhythm. Intravenous vernakalant appears to be especially effective in treating short-duration AF. Budiodarone and oral vernakalant have phase II studies underway, and phase III studies are planned. XEN-D0101 is in early phase II, and NTC-801 is in early phase I. Finally, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) appear to be effective in the primary prevention of AF in patients with hypertension and HF and may be beneficial for secondary prevention in combination with established antiarrhythmic drugs.
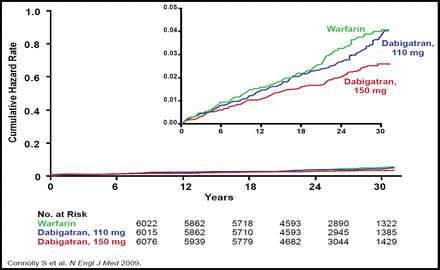
Novel anticoagulants may also enhance the management of AF. Dabigatran, the only oral direct thrombin inhibitor that is in late-stage development, may provide an important alternative to warfarin therapy in patients with AF. In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, 18,113 patients with AF and a risk of stoke were randomly assigned to receive treatment with dabigatran (110 mg or 150 mg) or warfarin, which was adjusted at least monthly to an INR of 2.0 to 3.0. The primary endpoint was stroke or systemic embolism. After a median follow-up of 2.0 years, dabigatran 110 mg was associated with a similar rate of stroke or systemic embolism compared with warfarin (1.53% vs 1.69%; p<0.0001 for noninferiority) but a lower risk of major bleeding (2.71% vs 3.36%; p=0.003). By comparison, treatment with dabigatran 150 mg significantly lowered the risk of stroke or systemic embolism relative to warfarin (1.11% vs 2.69%; p<0.001 for superiority), with a comparable risk of major bleeding (3.11% vs 3.36%; p=0.31; Figure 3) [Connolly SJ et al. N Engl J Med 2009].
RE-LY Primary Outcome.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Numerous oral direct Factor Xa inhibitors are in various stages of clinical development, including rivaroxaban, apixaban, edoxaban, and betrixaban, and are anticipated to overcome many of the limitations of current options for anticoagulation (Table 1). These agents are also being evaluated in other indications, such as acute coronary syndrome and venous thromboembolism, and for use with mechanical valves. Other novel agents that show potential in the management of AF include TTP889, a factor IXa inhibitor, and tecarfarin, a vitamin K antagonist.
Selected Factor Xa Inhibitors in Development for the Management of AF.
Options for the pharmacological management of AF are numerous and rapidly expanding. Although restoration and maintenance of sinus rhythm remain a challenge with current agents, new antiarrhythmic options may improve long-term cardioversion and ultimately alter the natural history of AF. In addition, novel anticoagulants may reduce the risk of thrombotic events while minimizing the risk of bleeding, especially in high-risk populations.
- © 2009 MD Conference Express