Summary
New options, combined with existing technology for cardiac imaging, including computed tomography coronary angiography, cardiovascular magnetic resonance, and single-photon emission computed tomography myocardial perfusion imaging, provide valuable information for the diagnosis and management of coronary artery disease and other cardiovascular conditions. Yet, each of these imaging techniques carries potential radiation-related risks for patients that must be weighed against the potential information that such tests might provide.
- Imaging Modalities
- Cardiac Imaging Techniques
- Radiography
New options, combined with existing technology for cardiac imaging, including computed tomography coronary angiography (CTCA), cardiovascular magnetic resonance (CMR), and single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI), provide valuable information for the diagnosis and management of coronary artery disease (CAD) and other cardiovascular conditions. Yet, each of these imaging techniques carries potential radiation-related risks for patients that must be weighed against the potential information that such tests might provide.
Computed Tomography Coronary Angiography
Given the increased availability of CTCA in the assessment of CAD, physicians must consider the responsible use of this valuable diagnostic tool, said Pamela K. Woodard, MD, Washington University School of Medicine, St. Louis, MO. The amount of ionizing radiation that is associated with CTCA is not negligible and may increase the long-term risk of cancer. The risk of CTCA-associated cancer is particularly high for women and younger patients and for combined cardiac and aortic scans. The lifetime cancer risk that is associated with a standard cardiac scan in a 20-year-old woman is 1 in 143, compared with 1 in 3261 for an 80-year-old man [Einstein AJ et al. JAMA 2007].
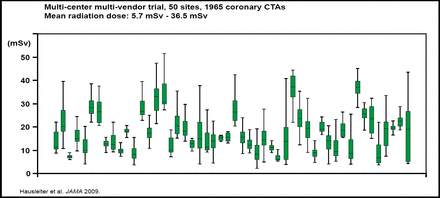
One way to minimize the risks that are associated with CTCA is to reduce the radiation exposure that is associated with the procedure. According to findings from the Prospective Multicentre Study on Radiation Dose Estimates of Cardiac CT Angiography in Daily Practice (PROTECTION 1), mean radiation doses that were associated with CTCA were highly variable, ranging from 5.7 to 36.5 mSv (Figure 1). In addition, radiation doses were affected by factors, such as patient weight, the absence of stable sinus rhythm, scan length, and type of 64-slice CT system [Hausleiter J et al. JAMA 2009]. Strategies to reduce radiation dose do not appear to compromise image quality, and therefore dose-saving strategies are recommended for selected patients. Education of cardiologists, radiologists, and CT technicians is also required to help minimize radiation exposure.
Radiation Doses Associated with CTCA: PROTECTION 1.
Copyright © 2009 American Medical Association. All rights reserved.
Restricting the use of CTCA to select patient populations may also reduce the risk of excess radiation exposure, Dr. Woodard said. For example, CTCA is appropriate for the triage of patients with acute chest pain and in the assessment of patients with inconclusive stress test results. In the Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT I) trial, early CTCA significantly improved the diagnosis of patients with acute chest pain and low to intermediate risk of acute coronary syndrome (ACS) in the emergency department [Hoffmann U et al. J Am Coll Cardiol 2009]. In a 2-year prospective study, the use of CTCA provided sufficient diagnostic information to allow the majority of patients with suspected CAD to avoid invasive coronary angiography (ICA) [Abidov A et al. J Nucl Cardiol 2009].
By comparison, the role of CTCA in the assessment of patients with chronic chest pain is unclear. In the Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) trial, CTCA detected the presence of obstructive coronary stenosis at thresholds of 50% and 70% stenosis. CTCA had a very high negative predictive value (99%), indicating that it is an effective noninvasive alternative to ICA for ruling out obstructive coronary artery disease [Budoff MJ et al. J Am Coll Cardiol 2008]. However, findings from the Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography (CORE 64) trial indicated that the negative predictive value (83%) and positive predictive value (91%) of CTCA were not sufficient to replace conventional coronary angiography in patients with suspected CAD [Miller JM et al. N Engl J Med 2008]. Therefore, in the absence of outcome data to support the use of CTCA in patients with chronic chest pain and no known CAD, the benefits of CTCA may not outweigh the risks of radiation exposure in this population, Dr. Woodard said.
Ongoing comparative effectiveness trials may clarify the role of CTCA in the assessment of specific patient groups. The ROMICAT II study and a trial from the American College of Radiology Imaging Network (ACRIN PA 4005) will assess CTCA in the management of acute chest pain in the emergency department, while the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial will evaluate CTCA in the outpatient chronic chest pain setting.
Cardiovascular Magnetic Resonance
Dudley Pennell, MD, Imperial College, London, UK, described CMR as an important diagnostic tool in the management of cardiomyopathy, heart failure, and other cardiac conditions. For example, CMR is increasingly used as an alternative to routine ICA in the assessment of dilated cardiomyopathy (DCM). In this setting, CMR is highly effective in excluding the presence of left ventricular (LV) dysfunction that is related to CAD in patients with heart failure. CMR is also able to improve the optimal management of DCM by distinguishing between two distinct subgroups of DCM patients, including those who have fibrosis that involves the midportion of the ventricle and those who have an infarction pattern of enhancement and require further evaluation for CAD [McCrohon JA et al. Circulation 2003].
CMR is associated with important diagnostic advantages compared with traditional echocardiography in the assessment of hypertrophic cardiomyopathy (HCM). In particular, CMR is able to identify regions of LV hypertrophy that are not readily recognized by echocardiography, particularly in the anterolateral LV free wall [Rickers C et al. Circulation 2005]. CMR also enhances the diagnosis and management of patients with various infiltrative disorders, such as cardiac amyloidosis and myocardial siderosis in patients with thalassemia [Maceira AM et al. Circulation 2005; Modell B et al. J Cardiovasc Magn Reson 2008].
Appropriateness Criteria in Cardiac Imaging
Given concerns about the rapid growth of cardiac imaging studies, several societies have developed appropriate use criteria to guide effective utilization of imaging studies in clinical practice. In 2005, the American College of Cardiology Foundation (ACCF) and American Society of Nuclear Cardiology (ASNC) developed appropriateness criteria for SPECT MPI [Brindis RG et al. J Am Coll Cardiol 2005]. In 2006, the American Heart Association officially endorsed the ACCF/ASNC criteria for SPECT MPI [Gibbons R et al. Circulation 2006].
Currently, the American College of Cardiology (ACC) and American College of Radiology (ACR) are collaborating on the development of evidence-based multimodality appropriateness criteria. The ACC/ACR criteria will address the appropriate use of imaging in the management of acute chest pain and heart failure, said Leslee J. Shaw, PhD, Emory University, Atlanta, GA. In developing these criteria, the ACC and ACR will take into account recent data on the estimated radiation dose and cancer risk that are associated with specific scenarios, such as CT scanning for coronary artery calcification (CAC) screening. In 2009, Kim and colleagues estimated that the excess lifetime cancer risk that is associated with a single CT scan to assess CAC is 8 cases per 100,000 men and 20 cases per 100,000 women. Assuming CAC screening every 5 years from age 45 to 75 years for men and age 55 to 75 years for women (a “worst case” scenario), the estimated excess lifetime cancer risk increases to 42 cases per 100,000 men and 62 cases per 100,000 women [Kim KP et al. Arch Intern Med 2009]. Updated estimates of imaging-related risk will be invaluable for developing future appropriate use criteria, Dr. Shaw concluded.
- © 2009 MD Conference Express