Summary
Results from the Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies [ARBITER-6 HALTS; NCT00397657] trial showed that combination therapy that uses a statin plus niacin is more effective than the combination of a statin plus ezetimibe in reducing carotid intima-media thickness.
- Lipid Disorders Clinical Trials
Results from the Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies (ARBITER-6 HALTS; NCT00397657) trial, presented by Allen J. Taylor, MD, Walter Reed Army Medical Center, Washington, DC, showed that combination therapy that uses a statin plus niacin is more effective than the combination of a statin plus ezetimibe in reducing carotid intima-media thickness (CMIT).
The ARBITER-6 HALTS trial enrolled a total of 363 subjects (mean age 65 years; 80% men) with coronary heart disease (CHD) or a CHD risk equivalent who were receiving long-term (6±5 years) statin therapy (95% simvastatin or atorvastatin; mean dosage 42±25 mg/day) and who had an LDL-C level <100 mg/dL (mean 82.1±23.1 mg/dL) and an HDL-C level <50 mg/dL (<55 mg/dL for women). Subjects were randomly assigned in a double-blind fashion to receive open-label extended release (ER) niacin (target dosage 2000 mg/day) or ezetimibe (10 mg/day) along with their usual statin. The primary study endpoint was the between-group difference in the change from baseline in mean CMIT after 14 months. Secondary endpoints included change in lipid values, a composite of major adverse cardiovascular events (MACE; ie, myocardial infarction, myocardial revascularization, hospital admission for an acute coronary syndrome, and death from CHD), adverse event (AE)-associated discontinuations, and health-related quality of life (HRQoL).
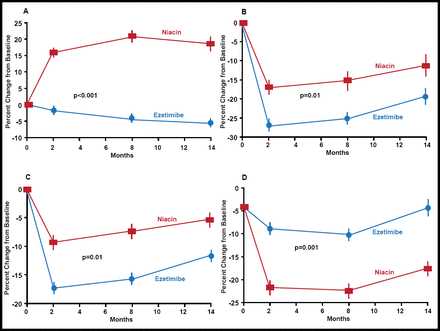
The study was stopped prematurely, based on the consistency of findings in CMIT at 8 and 14 months; results of a sensitivity analysis, and a post hoc analysis that suggested potentially paradoxical effects of ezetimibe, after 208 patients (111 ezetimibe; 97 niacin) had completed the trial. When compared with baseline, the addition of niacin to statin therapy resulted in a significant regression of both mean and maximal CMIT at 8 and 14 months; the corresponding changes in CMIT with ezetimibe compared with baseline were not significant on either measure at either timepoint. At 14 months, the between-group comparisons were significant for both measures: −0.0142 mm±0.0041 reduction in mean CMIT for the niacin combination versus −0.0007 mm±0.0035 with ezetimibe (p=0.01) and −0.0181 mm±0.0050 versus −0.0009 mm±0.0039, ezetimibe and niacin combinations, respectively (p=0.006). Mean HDL-C levels in the niacin group increased by 7.5±9.2 mg/dL, while those in the ezetimibe group decreased by 2.8±5.7 mg/dL (p<0.001). Mean LDL-C levels decreased by 17.6±20.1 mg/dL in the ezetimibe group and by 10.0±24.5 mg/dL (p=0.01) in the niacin combination group. There was a significant reduction in triglyceride levels in both groups (Figure 1).
Mean Percent Changes in Cholesterol and Triglyceride Levels.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
The incidence of MACE was significantly (p=0.04) higher in the ezetimibe group (5%; 9/165) compared with the niacin group (1%; 2/160). AE-related withdrawals were similar in the two groups. Adherence was significantly (p<0.001) higher in the ezetimibe group (95±8%) compared with the niacin group (88±15%). Cutaneous flushing was reported in 36% of patients in the niacin group. The final dosage of ER niacin was 2000 mg/day in 75%, 1500 mg/day in 3%, 1000 mg/day in 12%, and 500 mg/day in 10% of patients. HRQoL outcomes were similar in both groups.
A number of limitations of this study must be considered. ARBITER-6 HALTS was a small open-label trial that used a controversial surrogate endpoint, and thus these data can not be used to evaluate clinical benefit adequately. The study was prematurely terminated (which may have exaggerated the observed benefits), and the post hoc analysis that suggested a paradoxical effect with ezetimibe with possible adverse clinical consequences was neither robust nor well supported by external data. Several large ongoing randomized double-blinded clinical trials are evaluating the clinical benefit and safety of niacin and ezetimibe (although none of these trials is comparing the two directly). Such large-scale trials will serve as the foundation for shaping future guidelines and clinical practice with niacin and with ezetimibe.
- © 2009 MD Conference Express