Summary
Pharmacogenetics, the study of the interaction between genes and drug therapy, has been proposed as a way to “personalize” medical therapy. It is a routine and expanding application in oncology. This article provides overview of progress to date for the use of pharmacogenetics in cardiovascular medicine and what is yet to be done.
- Cardiology Genomics
Genetically driven personalized pharmacotherapy was one of the subjects that were discussed during the International Congress on Genetics and Genomics, during this year's AHA Scientific Sessions. Pharmacogenetics, the study of the interaction between genes and drug therapy, has been proposed as a way to “personalize” medical therapy. It is a routine and expanding application in oncology. During this session, researchers provided an overview of progress to date for the use of pharmacogenetics in cardiovascular medicine and what is yet to be done.
“Warfarin has been proposed as the first application for pharmacogenetics in cardiovascular medicine, primarily because of its high use, wide interindividual variation in dose requirement, narrow therapeutic range, and the frequent association of anticoagulants with adverse events and mortality,” said Jeffrey L. Anderson, MD, Intermountain Medical Center, Murray, UT. The initiation of warfarin therapy is particularly challenging, and it has been suggested that the addition of genetic testing might reduce the number of strokes and serious bleeds and result in significant health care savings [http://www.aei-brookings.org/publications/abstract.php?pid=1127].
Dr. Anderson discussed a few of the studies that have assessed warfarin pharmacogenetics. In one study that involved patients who were receiving warfarin and who had a stable INR (2 to 3) for >1 month, carriage of a single or double CYP2C9 variant reduced warfarin dose 18% to 72%; carriage of a q or 2 VKORC1 variant reduced it by 34% to 54%. At least one of these variants was shown to be present in 70% of the study population [Carlquist JF et al. J Thromb Thrombolysis 2006]. Results from another study (CoumaGen) that assessed pharmacogenetic-guided warfarin therapy and its impact on INR-based efficacy and safety showed that using pharmacogenetics to guide warfarin therapy did not reduce the percentage of out-of-range INR in the overall study population. However, it did improve the accuracy and efficiency of warfarin dose initiation, and results of a subset analysis indicated a reduction in the percentage of out-of-range INR for wild-type and multiple variant genotypes [Anderson JL et al. Circulation 2007]. A new study—CoumaGen II—with a larger patient population and a more refined algorithm has been initiated to follow up on these results. Several other studies that are evaluating the role of pharmacogenetic-guided warfarin therapy are ongoing or planned, including Clarify Optimal Anticoagulation Through Genetics (COAG; NCT00839657) and Genetics InFormation Trial of Warfarin Therapy (GIFT; NCT01006733).
“The use of pharmacogenetics to guide warfarin dosing is feasible,” said Dr. Anderson. “It better predicts the eventual maintenance dose, particularly in those with no or multiple variants, but we still don't know what the impact will be on patient outcomes, which will require additional clinical trials.”
“Everyone who cares for patients with heart failure (HF) or hypertension is aware of the great deal of variability in response to β-blockers,” said Amber Beitelshees, PharmD, University of Maryland School of Medicine, Baltimore, MD, “as evidenced by the difference in response in systolic and diastolic blood pressure in hypertensive patients and ejection fraction, and left ventricular end-diastolic volume in HF patients, as well as the high number of nonresponders in both types of patients.” Part of the reason for this variability is likely genetics, and it stems from a variety of areas in β-blocker pharmacology, such as the drug metabolism enzyme, the receptors themselves, the G-proteins to which the receptors couple, the G-protein receptor kinases, the enzymes that contribute to cyclic AMP formation, and the beta-arrestins. Key questions that concern β-blocker pharmacogenetics involve the portability of these associations across phenotypes and patient populations and whether they exhibit a class effect.
The ADRB1 gene is the most widely studied in β-blocker pharmacogenetics. Dr. Beitelshees reviewed a number of these studies, which show fairly consistent associations between ADRB1 Arg389Gly and both metoprolol and atenolol in antihypertensive response and with treatment outcomes with atenolol. In H F, ADRB1 is significantly associated with left ventricular function in response to metoprolol and carvedilol but not with treatment outcomes [Terra SG. Clin Pharmacol Ther 2005; Chen L. Pharmacogenet Genom 2007; Pancanowski MA et al. Clin Pharmacol Ther 2008]. Results from the BEST study indicate that ADRB1 is associated with improved survival in bucindolol-treated patients [Liggett SB et al. PNAS 2006]. So far, study results are inconsistent concerning whether there is a class effect.
Dr. Beitelshees said that she believes that there is not yet sufficient evidence of a role for genotyping in the treatment of hypertension or H F. More robust studies are needed before genotyping can be applied practically in the clinic.
As with the anticoagulants and β-blockers, there is also a variable response to clopidogrel, with one study that shows that ∼30% of patients are clopidogrel-resistant [Gurbel PA et al. Circulation 2003]. Marc Sabatine, MD, Brigham and Women's Hospital, Boston, MA, discussed how clopidogrel metabolism may contribute to this variability in response.
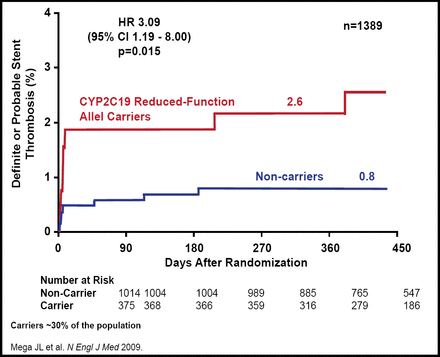
Clopidogrel is a prodrug that requires biotransformation by CYP450 enzymes into an active metabolite. About 85% of clopidogrel is degraded by esterases either in the intestinal lumen or the plasma. The remaining 15% undergoes two successive oxidative steps in the liver that are mediated by CYP450. Six genotypes are known to be associated with clopidogrel metabolism: CYP2C19, CYP2C, CYP2B6, CYP3A5, CYP3A4, and CYP1A2. Dr. Sabatine and colleagues have done extensive work that has examined the CY2C19 genotype. Approximately 30% of the population carries a reduced-function CYP2C19 allele. These individuals have been shown to have lower levels of the active metabolite of clopidogrel, less platelet inhibition, and higher rates of ischemic events and stent thrombosis (Figure 1) [Mega JL et al. N Engl J Med 2009]. Although there are some data that indicate that higher doses of clopidogrel may improve platelet inhibition in carriers of reduced-function CYP2C19 alleles, an option for patients who carry this allele may be prasugrel, a third-generation ADP receptor blocker with a different metabolism that does not appear to be affected by common CYP450 genetic variation [Gladding P et al. JACC 2008; Mega JL et al. Circulation 2009].
CYP2C19 and Stent Thrombosis.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
“What we are really trying to do with genetic testing is to discriminate who will have an outcome of one particular type versus another,” said Robert M. Califf, MD, Duke Clinical Research Institute, Durham, NC, in the closing presentation. “While there is no doubt that genes that alter the response to a drug can be found, it's rare that they provide a deterministic prediction of outcomes.” As such, the use of pharmacogenetic testing is a comparative effectiveness issue, and the value of the information that is obtained can be determined the same way as with any other diagnostic or prognostic information.
“While pharmacogenetic tests are promising and can provide information for medical decision-making, the clinical practice and reimbursement communities should be appropriately skeptical until the data are available. To avoid misinterpretation and misuse, randomized controlled trials are needed,” said Dr. Califf.
The COAG research network, which was mentioned by several speakers, is conducting collaborative research to assess whether the use of genetic and clinical information for selecting the initial dose of warfarin will lead to improved anticoagulant stability compared with using clinical information only. The network includes 12 clinical sites, a clinical trial coordinating center, a central drug distribution center, and a central laboratory. Dr. Califf is the Chair, and Dr. Anderson is a member of the COAG Steering Committee. The study is currently recruiting. Additional information at http://coagstudy.org.
- © 2009 MD Conference Express