Summary
Over the last 15 to 20 years, investigators have slowly started to unravel a fascinating link between psychiatric disorders and cardiovascular disease.
- Coronary Artery Disease
- Hypertensive Disease
- Thrombotic Disorders
- Mood Disorders
Over the last 15 to 20 years, investigators have slowly started to unravel a fascinating link between psychiatric disorders and cardiovascular disease. This link was the subject of several presentations at this year's Annual Meeting of the American Psychiatric Association in San Francisco, CA.
Stephen B. Woolley, DSc, Burlingame Center for Psychiatric Research and Education, The Institute of Living, Hartford, CT, reviewed the results of a recent population-based study that showed a significant increase in the risk of developing cardiovascular disease in individuals with schizophrenia compared with the nonpsychotic population. In a large community-based sample of subjects (n=3101) aged between 40 and 64 years, individuals with schizophrenia or schizophreniform disorder had a relative risk (RR) of 4.41 (95% CI, 1.41 to 13.79) of developing symptoms of ischemic heart disease and a nonsignificant RR of 1.58 (95% CI, 0.19 to 13.37) of having chest pains, even after demographics and psychiatric conditions (ie, depression and anxiety) were controlled for. Evidence was presented that suggested that smoking, poor diet, and low physical activity additionally confounded but did not explain the elevated risk of developing cardiovascular disease (CVD) symptoms, whereas substance abuse/dependence and other psychiatric conditions did not appear to further confound the associations. The metabolic syndrome, a cluster of risk factors that, when present together, increases the risk for CVD and type 2 diabetes, is highly prevalent among individuals with schizophrenia. Results of a recent review of the metabolic syndrome in schizophrenia patients support routine monitoring in this population and early intervention for the treatment and prevention of possible cardiovascular problems [Meyer JM & Stahl SM. Acta Psychiatr Scand 2009].
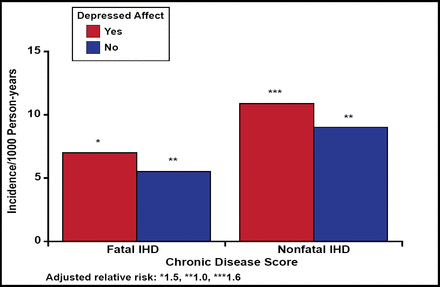
In the National Health Examination Follow-up Study, subjects (n=2832) with depression were shown to have a higher risk for cardiac events after 12.4 years of follow-up, despite having no history of ischemic heart disease at baseline. The RR was 1.5 (95% CI, 1.0 to 2.3) for those with depressed affect, 1.6 (95% CI, 1.0 to 2.5) for those with moderate levels of hopelessness, and 2.1 (95% CI, 1.1 to 3.9) for those with severe hopelessness (Figure 1) [Anda R et al. Epidemiology 1993].
Fatal and Nonfatal Ischemic Heart Disease in Persons with Depressed and Nondepressed Affect.
Anda R et al. Epidemiology 1993.
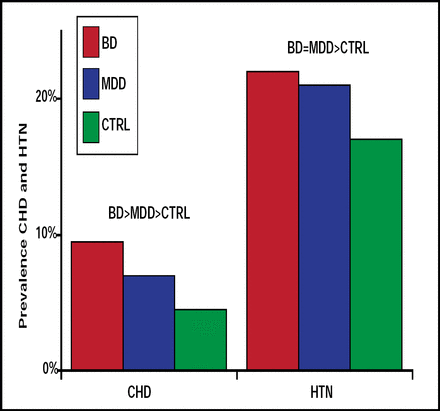
Ischemic heart disease is also common among persons who seek treatment for bipolar disorder and, along with another common symptom—hypertension—contributes to increased treatment costs. Data from the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions, presented in a poster by Benjamin I. Goldstein, MD, University of Pittsburgh, Pittsburgh, PA, showed a significantly (p<0.001) greater prevalence of ischemic heart disease (OR 4.95) and hypertension (OR 2.34) among subjects with bipolar disorder compared with controls (Figure 2). Subjects with bipolar disorder and ischemic heart disease or hypertension were also an average of 14 and 13 years younger, respectively, than controls with ischemic heart disease and hypertension.
Prevalence of Coronary Heart Disease and Hypertension.
Benjamin Goldstein B. NR4-057. APA 2009.
Depression may lead to the development of CVD through its association with the metabolic syndrome. In women, a history of major depression has been shown to predict increased risk of developing the metabolic syndrome (HR 1.66; 95% CI, 0.99 to 3.75) [Goldbacher EM et al. Psychosom Med 2009]. Among middle-aged women, depression is significantly (p<0.05) and consistently associated with obesity, lower physical activity, and higher caloric intake [Simon GE et al. Gen Hosp Psychiatry 2008], as well as diabetes [Anderson RJ et al. Diabetes Care 2001]. Men with moderate/severe depression (≥48 on the Sheehan Disability Scale [SDS]) at baseline have been shown to have a significantly higher risk of having type 2 diabetes after 8 years of follow-up compared with those who were not depressed (≤39 on the SDS; p<0.05), even after other known risk factors were controlled for [Kawakami N et al. Diabetes Care 1999].
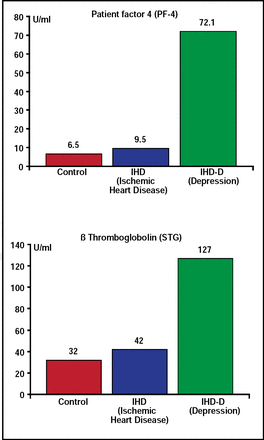
There are many theories to explain the biological mechanisms that underlie the relationship between depression and CVD. Charles B. Nemeroff, MD, PhD, Emory University School of Medicine, Atlanta, GA, reviewed some of these mechanisms. One major candidate is the platelet clotting cascade—specifically, whether depressed patients have a clotting diathesis (ie, are they more likely to form a thrombus than nondepressed patients). Mean platelet factor 4 and beta-thromboglobulin plasma levels in patients with ischemic heart disease and comorbid depression are significantly higher than those who are not depressed (Figure 3) [Laghrissi-Thode F et al. Biol Psychiatry 1997]. Increased platelet activation in depressed patients with ischemic heart disease can be partially reversed by selective serotonin reuptake inhibitors (SSRIs) [Serebruany VL et al. Am J Psychiatry 2005].
Another possible mechanism is increased inflammation in depressed patients, particularly major inflammation of the vessel wall. Patients with major depressive disorder (MDD) have been found to exhibit increased peripheral blood inflammatory biomarkers, including inflammatory cytokines, which have been shown to interact with virtually every pathophysiological domain that is known to be involved in depression (eg, neurotransmitter metabolism, neuroendocrine function, and neural plasticity) and to play a role in CVD, as well.
Depression in patients with increased inflammatory biomarkers is more likely to be treatment-resistant. Preliminary data from patients with inflammatory disorders, as well as medically healthy depressed patients, suggest that inhibiting proinflammatory cytokines or their signaling pathways may improve depressed mood and increase treatment response to conventional antidepressant medication [Miller AH et al. Biol Psychiatry 2009]. These results indicate that depression and cardiovascular disorders may share a common pathogenesis—stress—leading to an inflammatory cascade.
Ischemic Heart Disease and Depression: Platelet Activation.
Laghrissi-Thode F et al. Biol Psychiatry 1997.
Lawson Wulsin, MD, University of Cincinnati, Cincinnati, OH, said that in his opinion, clinical evidence points to autonomic imbalance as the mechanism that has the most promise for explaining the connection between psychiatric disorders and cardiovascular disease. He discussed the results of several studies that indicated that dysfunction in the autonomic nervous system is associated with depression.
Heart rate variability is significantly lower in depressed than nondepressed patients (90 ± 35 vs 117 ± 26 ms; p≤0.01), even after adjusting for relevant covariates [Carney RM et al. Am J Cardiol 1995]. Patients with MDD have a significantly lower Valsalva ratio and maximum/minimum ratio and greater sympathovagal balance than healthy controls, indicating decreased parasympathetic and increased sympathetic activity [Udupa K et al. J Affect Disord 2007]. Decreased vagal function is associated with increased cardiovascular morbidity and mortality that are independent of traditional ischemic heart disease risk factors, while risk reduction is associated with increased vagal function. In depressed patients who have survived the acute phase of a myocardial infarction, the SSRI sertraline facilitates the rate of recovery [McFarlane A et al. Am Heart J 2001]. Abnormalities of autonomic tone, as evidenced by lower heart rate variability, may be partly responsible for the higher rate of atrial fibrillation, coronary heart disease, cardiac death, and overall mortality that is seen in patients with the metabolic syndrome [Gehi AK et al. J Cardiovasc Electrophysiol 2009]. Further evidence for this connection is seen in data that show that elevated total cholesterol is a significant predictor of treatment nonresponse and lack of remission to antidepressant treatment [Iosifescu DV et al. Psychosom Med 2005].
Dr. Wulsin believes that studies that use interventions that raise vagal tone (exercise, relaxation training, biofeedback, psychotherapy, acupuncture, vagus nerve stimulation, transcranial magnetic stimulation, omega-3 supplements, beta-blockers, and SSRIs) need to be done to assess whether treating autonomic imbalance can reduce the risk of onset of metabolic syndrome or prevent ischemic heart disease, particularly in patients with chronic psychiatric conditions that contribute to autonomic imbalance over many years.
The growing evidence that depression is a precursor for an increased risk of cardiac morbidity and mortality in medically healthy individuals supports a strategy of increased awareness and screening. Primary prevention (aerobic exercise, nutritional counseling, mindfulness, meditation, and web-based cognitive behavioral therapy) and secondary prevention (adequate management of hypertension, diabetes, and dyslipidemia) strategies should be considered for patients who are diagnosed with depression. Post event interventions in myocardial infarction patients have the potential to enhance psychosocial recovery and decrease morbidity, mortality, and overall health care costs.
- © 2009 MD Conference Express