Summary
Major depressive disorder (MDD) affects about 15 million adults in the United States. Within that population, only one-third of patients who are treated with first-line antidepressant therapies achieve remission [Trivedi MH, Papakostas G, Perlis R. APA 2009]. Therefore, therapeutic augmentation, the addition of nonantidepressant compounds to an antidepressant regimen, may be a more reasonable option for those patients with first-line treatment resistance. This article explores the issue of treatment resistance in MDD and possible management strategies.
- Psychopharmacology
- Mood Disorders
Major depressive disorder (MDD) affects about 15 million adults in the United States. Within that population, only one-third of patients who are treated with first-line antidepressant therapies achieve remission [Trivedi MH, Papakostas G, Perlis R. APA 2009]. Therefore, therapeutic augmentation, the addition of nonantidepressant compounds to an antidepressant regimen, may be a more reasonable option for those patients with first-line treatment resistance. Several experts in the field explored the issue of treatment resistance in MDD and possible management strategies at the American Psychiatric Association annual meeting.
Roy Perlis, MD, MSc, Massachusetts General Hospital, Boston, MA, discussed possible predictors and second-line approaches for MDD patients with differential responses to antidepressant monotherapy. He pointed out that there are 2 kinds of predictors that are related to inadequate response: nonspecific predictors that are related to poor overall response (ie, greater depression severity, socioeconomic status, gender, and level of anxiety or irritability) and drug-specific predictors (ie, decreased SSRI response but adequate SNRI response) [Cohen A et al. Arch Gen Psychiatry 2006; Trivedi MH et al. Am J Psychiatry 2006; Fava M et al. Am J Psychiatry 2008]. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, higher levels of anxiety and irritability in MDD patients who were treated with either monotherapy or an augmented antidepressant regimen were associated with poorer treatment outcomes (p<0.05) [Fava M et al. Am J Psychiatry 2008]. Based on these results, Prof. Perlis said, “People with higher levels of irritability or anxiety during depression probably merit particular attention, because their likelihood of remitting is lower than those without these symptoms.”
Treatment-emergent suicidal ideation also remains a problem in the management of MDD. Prof. Perlis discussed possible biomarkers that are related to this issue. In a study by Laje and colleagues, 2 biomarkers within GRIK2 and GRIA3 (rs4825476 and rs2518224) were associated with treatment-emergent suicidal ideation during citalopram therapy (rs4825476 permutation p=0.01; rs2518224 permutation p=0.003) [Laje G et al. Am J Psychiatry 2007]. In a study by Perlis and colleagues, polymorphisms that span cyclic adenosine monophosphate response element-binding (CREB1) protein were associated with treatment-emergent suicidality among men with depression [Perlis RH et al. Arch Gen Psychiatry 2007]. However, these studies have not yet been replicated, so further investigation is warranted.
There are some possible biomarkers that may predict treatment resistance and poor response. There are commercially available tests for poor metabolizers that involve cytochrome p450 variation, particularly in patients with multiple instances of intolerance at low doses or nonresponse despite aggressive dosing. Prof. Perlis emphasized that there is no evidence that these tests are useful for choosing antidepressants; they are more expensive than standard blood level testing; and they only test 1 factor and do not take into account other variables, such as diet, adherence, and other comorbidities. Therefore, blood levels are more clinically useful.
Genomewide association studies have also investigated antidepressant efficacy in MDD, but no single gene has consistently appeared at the top of the list for antidepressant response across studies. “Most likely when we get there, it will be with a combination of many genes rather than a single gene,” said Prof. Perlis.
Mudhukar Trivedi, MD, University of Texas, Southwestern Medical Center, Dallas, TX, discussed various augmentation strategies prior to and in conjunction with the STAR*D trial. Lithium augmentation has the most evidence with regard to overall response (p=0.0001) and rapid response rate, although most of the published studies are small [Crossley NA & Bauer M. J Clin Psychiatry 2007; Thase ME, Rush AJ. Psychopharmacology: The Fourth Generation of Progress, 1995]. Augmentation with triiodothyronine (T3) has also had promising results. A meta-analysis of 8 T3 augmentation trials by Aronson and colleagues demonstrated that patients who were treated with T3 augmentation were twice as likely to respond as controls (OR, 2.09; 95% CI, 1.31 to 3.32; p=0.002) [Aronson R. Arch Gen Psychiatry 1996]. Additionally, augmentation with buspirone has demonstrated safety and efficacy [Spier S. Depress Anxiety 1998; Lam R et al. J Clin Psychiatry 2004]. However, the published T3 and buspirone studies were small, and results were inconsistent.
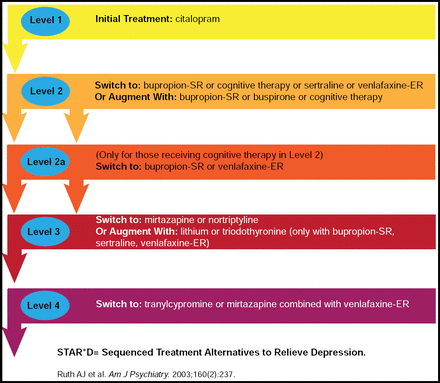
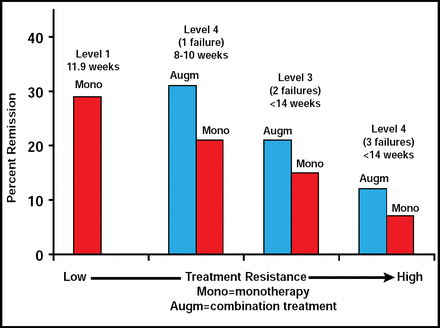
The STAR*D study was a large trial that consisted of 4 levels of treatment, beginning with citalopram only and moving to alternative treatment or augmentation depending on patient response (Figure 1). The STAR*D remission rates (based on HAM-D-17<8) for all levels of the study were slightly better for patients who were undergoing augmentation versus monotherapy [McGrath PJ et al. Am J Psychiatry 2006; Rush AJ et al. Am J Psychiatry 2006; Nierenberg AA et al. Am J Psychiatry 2006; Trivedi MH et al. J Clin Psychiatr. 2006; Trivedi MH et al. N Engl J Med 2006]. A one-third remission rate was noted in patients who were undergoing augmentation therapy in levels 2 and 3 of the study (Figure 2). Prof Trivedi said that although the rates of remission were modest in STAR*D, the study demonstrated the need for a multitiered approach, more aggressive treatment options, and measurement-based care.
STAR*D Treatment Algorithm Snapshot.
STAR*D Clinical Study Results.
Other potential augmentation strategies include mirtazapine, psychostimulants, folic acid, and estrogen therapies. A folic acid augmentation study by Coppen and Bailey demonstrated improvement in Hamilton Rating Scales in the fluoxetine plus folic acid group versus placebo. This was confined to women, in whom the mean Hamilton Rating Scale score on completion was 6.8 (S.D. 4.1) in the fluoxetine plus folate group, as compared with 11.7 (S.D. 6.7) in the fluoxetine plus placebo group (p<0.001) [Coppen A, Bailey J. J Affect Disord 2000]. There was no significant change in men. The folic acid and other augmentation treatments have been investigated in very small studies to date, and although the preliminary data have been promising with regard to safety and efficacy, larger randomized, controlled trials are needed to establish their feasibility as augmentation alternatives for MDD.
Prof. Trivedi also noted that residual symptoms, such as insomnia and fatigue, are problems for those who do not attain remission, and it is important to select agents that may match/treat residual symptoms. Residual symptoms are associated with an increased risk for relapse and increased psychosocial impairments.
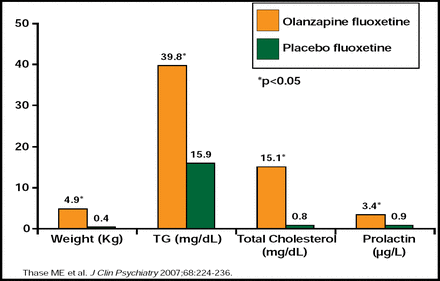
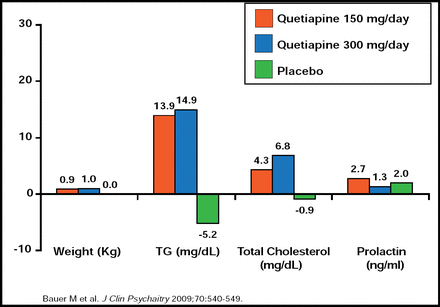
George Papakostas, MD, Massachusetts General Hospital, Boston, MA, discussed the use of atypical antipsychotics in the management of MDD. Risperidone, quetiapine, olanzapine, and aripiprazole have been investigated as possible augmentation agents for patients with MDD, but it is important to balance the risks and benefits of these treatment strategies [Papkostas GI et al. J Clin Psychiatry 2007]. Thirty-seven percent of patients within these trials discontinued the augmentation component of therapy due to intolerance (p<0.05) [Papkostas GI et al. J Clin Psychiatry 2007]. Weight gain was significantly higher among patients who received either risperidone (a difference of 2.5 to 4.0 lbs in 4 weeks vs placebo) or aripiprazole (mean change from baseline at 6 weeks, 3.7 lbs) versus placebo [Keitner GI et al. J Psychiatric Res 2009; Berman R et al. J Clin Psychiatry 2007; Marcus R et al. J Clin Psychopharmacol 2008]. Significant changes in metabolic and endocrine parameters were noted with quetiapine and olanzapine (p<0.05 for both; Figures 3 and 4) augmentation [Thase ME at al. J Clin Psychiatry 2007; Bauer M et al. J Clin Psychiatry 2009]. Other tolerability issues that were associated with aripiprazole augmentation included akathisia, restlessness, insomnia, fatigue, blurred vision, and constipation. Somnolence, hypersomnia, edema, and dry mouth were associated with olanzapine augmentation.
Olanzapine Augmentation in TRD: Changes in Metabolic and Endocrine Patterns.
Quetiapine Augmentation in TRD: Changes in Metabolic and Endocrine Patterns.
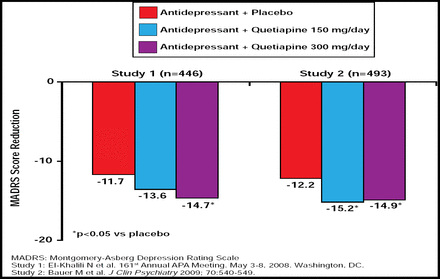
Prof. Papakostas also presented the evidence-based benefits of augmentation therapy that were related to atypical antipsychotics. In a study by Shelton and colleagues, 28 treatment-resistant MDD patients were randomized to receive either fluoxetine (mean 52 mg daily) plus placebo, olanzapine (mean 12.5 mg daily) plus placebo, or olanzapine plus fluoxetine (mean 13.5 mg/52 mg daily). Although this was a small study, patients in the olanzapine plus fluoxetine arm showed rapid clinical improvement, and olanzapine plus fluoxetine demonstrated superior efficacy for treating resistant depression compared with either agent alone (p<0.05) [Shelton RC et al. Am J Psychiatry 2001]. Three larger studies compared aripiprazole augmentation with placebo, resulting in a 26% to 36.8% remission rate for the aripiprazole augmentation groups (p<0.05) [Berman R, et al. J Clin Psychiatry 2007; Marcus R et al. J Clin Psychopharmacol 2008; Berman R et al. CNS Spectrums 2009]. Results of 2 randomized, double-blind, placebo-controlled trials that compared quetiapine (150 mg/day and 300 mg/day) augmentation versus placebo demonstrated significant MADRS score reduction in the quetiapine groups (p<0.05; Figure 5) [Bauer M et al. J Clin Psychiatry 2009; El-Khalili N et al. 161st Annual APA Meeting; May 2008].
Quetiapine Augmentation: Results of Two Randomized, Double-Blind, Placebo-Controlled Trials.
Aripiprazole and olanzapine are the first agents to receive an indication from the Food and Drug Administration as adjunctive therapy for treatment-resistant MDD, although further research that investigates long-term efficacy, safety, and tolerability is needed with regard to the use of atypical antipsychotics as augmentation therapy. “Clearly, even though this appears to be a promising strategy,” said Prof. Papkostas, “I think we have a ways to go in terms of further delineating the clinical niche and the clinical properties of this treatment strategy.”
Therapeutic augmentation for treatment-resistant MDD is a complex approach that is still in its clinical infancy. There are many exciting new options and potential tools that may assist physicians in further enhancing outcomes through individualized treatment, but this issue merits further investigation before a specific clinical regimen can be established.
- © 2009 MD Conference Express