Summary
The impact of attention deficit hyperactivity disorder (ADHD) extends beyond the symptoms of hyperactivity and inattentiveness. Approximately one-quarter of children with ADHD are reported as having emotional problems, expressed as temper outbursts, mood lability, and dysphoria [American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 2000], all of which impair the quality of life of the child and his family [Escobar R et al. Pediatrics 2005; Klassen AF, Miller A, Fine S. Pediatrics 2004].
- Child & Adolescent Neurodevelopmental Disorders
Attention deficit hyperactivity disorder (ADHD) is characterized by a persistent pattern of inattention and/or hyperactivity/impulsivity [American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 2000]. It is one of the most common psychiatric disorders of childhood, estimated to affect approximately 8% to 12% of children worldwide [Biederman J, Faraone SV. Lancet 2005]. The impact of ADHD extends beyond the symptoms of hyperactivity and inattentiveness. Approximately one-quarter of children with ADHD are reported as having emotional problems, expressed as temper outbursts, mood lability, and dysphoria [American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 2000], all of which impair the quality of life of the child and his family [Escobar R et al. Pediatrics 2005; Klassen AF, Miller A, Fine S. Pediatrics 2004].
To date, stimulants that are used to treat ADHD have been short-acting, requiring multiple-daily dosing, thus having the potential for uneven symptom control and abuse [Faraone SV. Expert Opin Pharmacother 2008; Stein MA. Am J Manag Care 2004]. Lisdexamfetamine dimesylate (LDX) is the first long-acting, prodrug stimulant that is indicated for the treatment of ADHD in children and adults. It is converted to L-lysine and active d-amphetamine after oral ingestion [Biederman J et al. Clin Ther 2007]. While a small amount of LDX is hydrolyzed to d-amphetamine in the gastrointestinal tract, the majority of LDX conversion takes place in the blood, which accounts for its improved bioavailability [Pennick M. Society of Biologic Psychiatry 2009].
Previous trials have evaluated the efficacy and safety of LDX in clinical [Biederman J et al. Clin Ther 2007; Findling RL, Childress A, Krishnan S, McGough JJ. CNS Spectr 2008] and laboratory classroom settings [Biederman J et al. Biol Psychiatry 2007; Wigal SB et al. American Academy of Child and Adolescent Psychiatry 2008]. In an analog classroom setting, LDX at doses of 20–70 mg/day yielded significant improvements in ADHD symptoms versus placebo up to 13 hours postdose [Biederman J et al. Biol Psychiatry 2007; Wigal SB et al. American Academy of Child and Adolescent Psychiatry 2008].
A number of posters at the 162nd Annual Meeting of the America Psychiatric Association in San Francisco, CA, presented additional data from previously reported studies regarding LDX treatment of ADHD in children aged 6 to 12 years.
NR2–019 Alain Katic, MD, Claghorn Research Clinic, Bellaire, TX
Improvement in emotional expression in children with attention deficit/hyperactivity disorder treated with 20 to 70 mg/day lisdexamfetamine dimesylate
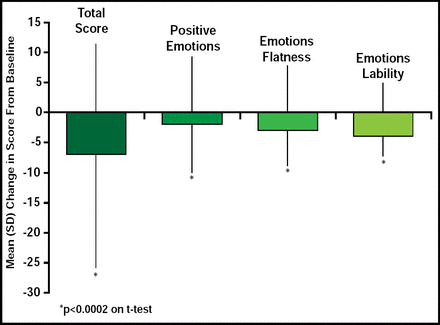
Stimulants may cause blunting of emotional expression in some patients [Kratochvil CJ et al. J Child Adolesc Psychopharmacol 2007; Perwien AR et al. J Atten Disord 2008]; however, in a recent study of LDX, treatment was associated with a small but statistically significant improvement in Expression and Emotion Scale for Children (EESC)a total and subscale scores [Findling RL et al. American Academy of Child and Adolescent Psychiatry 2008]. In a post hoc analysis of data from the latter study open-label, 7-week, dose optimization study, children (n=316) who were administered 20 mg/day to 70 mg/day of LDX showed a significant (p<0.0001) improvement in ADHD Rating Scale IV (ADHD-RS-IV)b scores (69.3% ± 23.3) at study endpoint. Mean change from baseline for the EESC total score was −7.4 ± 18.3 for positive emotions (p=0.0002), −2.4 ± 7.7 for emotional flatness (p<0.0001), and −2.8 ± 5.2 for emotional lability (p<0.0001; Figure 1), indicating that LDX treatment reduces ADHD symptoms with slight but clinically significant improvements in emotional expression.
Mean EESC Total and Subtotal Score Changes From Baseline at End of Study (n=304).
NR2–029 Thomas W. Frazier, Children's Hospital, Cleveland Clinic, Cleveland, OH
Duration of effects of lisdexamfetamine dimesylate on behavior of children with attention deficit/hyperactivity disorder in naturalistic settings
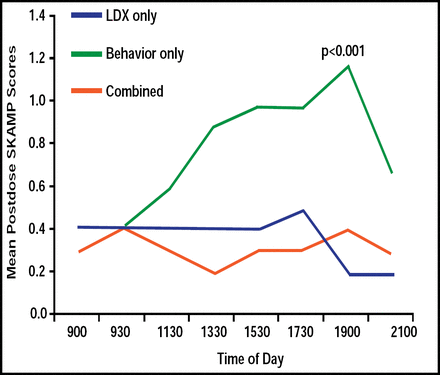
Little data are available regarding the effectiveness of long-acting stimulants compared with behavioral therapy to treat ADHD in natural settings. Some studies demonstrate the superiority of medications, whereas others suggest that behavioral and medications treatments are equivalent [Pelham WE Jr, Fabiano GA. J Clin Child Adolesct Psychol 2008]. Using the Swanson, Kotkin, Agler, M-Flynn (SKAMP)c, and Clinical Global Impressions-Severity (CGI-S)b rating scales, children (n=25) who were treated with LXD alone or in combination with behavioral therapy in a natural setting (home and school) had reduced ADHD symptoms and improvement in behavior. LDX only and LDX that was combined with behavioral therapy were more effective than behavioral therapy alone in maintaining SKAMP scores for up to 12.5 hours. LDX versus behavioral therapy effect size was 1.22. SKAMP scores deteriorated by 11:30 AM (p<0.001) in children who were treated with behavioral therapy alone (Figure 2). Children who were treated with LDX alone or in combination with behavioral therapy demonstrated better ability to follow instructions versus behavioral therapy alone at the 7:00 PM assessment time (p=0.03), whereas no differences were seen in the ability of children to calm themselves or tolerate frustration at a similar time point.
SKAMP Scores from 9:00 AM to 9:00 PM for Children (n=17) Administered LDX, Behavior, or Combined Regimens.
NR2–032 John Giblin, MD, Clinical Study Centers LLC, Little Rock, AR
Effect of lisdexamfetamine dimesylate on sleep in children aged 6 to 12 years with attention deficit/hyperactivity disorder
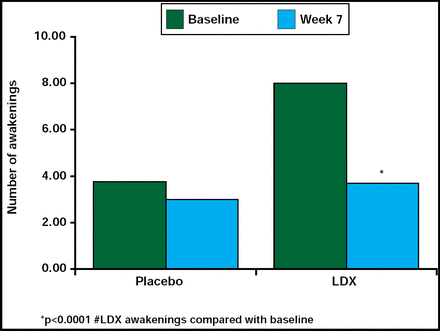
Treatment with psycho stimulants has been associated with sleep disturbances, including sleep onset and sleep maintenance problems in children with ADHD [Mindell JA, Owens JA. A clinical guide to pediatric sleep: Diagnosis and Management of Sleep Problems. Lippincott Williams and Wilkins 2003; Corkum P et al. J Am Acad Child Adolesc Psychiatry 1999]. Contrary to these findings, this study reported that LDX did not contribute to sleep problems, and there was some suggestion that LDX may even enhance sleep efficiency. In a randomized, double-blind, placebo-controlled, parallel-group, dose optimization study in children (n=24), LDX (30 mg/day to 70 mg/day) did not significantly (p=0.352) increase the latency to persistent sleep time compared with placebo, nor were the secondary endpoint measures of wake time after sleep onset and total sleep time significantly different between the 2 treatment groups, as measured by polysomnography and actigraphy. However, there was a significant decrease (p<0.0001) in the number of awakenings after sleep onset for the LDX-treated group compared with the placebo group (Figure 3). Sleep efficiency was increased relative to baseline in the LDX-treated group, but the difference did not reach statistical significance. Results from a sleep questionnaire that was filled out by parents/caregivers supported the data above. These findings suggest that LDX does not contribute to sleep disturbances in this population of children.
Effect of LDX on Number of Awakenings After Sleep Onset.
NR2–033 Lawrence Ginsberg, MD, Red Oak Psychiatry Associates, Houston, TX
Parental evaluation of lisdexamfetamine dimesylate in the treatment of children with attention deficit/hyperactivity disorder
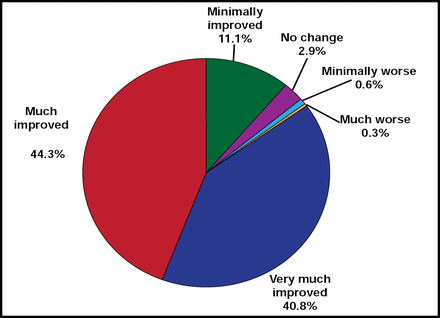
Reports of parental satisfaction with an ADHD treatment reflect parent perceptions of treatment outcomes and appear to provide informative, reliable, and accurate outcome measures [Biederman J et al. Pediatrics 2004; Bukstein OG. Am J Manag Care 2004]. In a 7-week, open-label, dose optimization trial of LDX (20 mg/day to 70 mg/day), a high percentage of parents of ADHD children (n=316) reported improved behavior and satisfaction with the treatment. The majority thought LDX was better than their child's previous therapy and said they were likely to continue with the treatment. Clinicians, using CG-I scores to measure change in ADHD, rated 284 (89.9%) subjects improved. Mean relative improvement in ADHD-RS-IV total score from baseline at endpoint was 69.3% ± 23.3 (p<0.0001). From Weeks 1 through 7, the percentage of subjects who were assessed as improved by their parents using the Parent Global Assessment (PGA)d scale increased from 29.3% to 85.0% (Figure 4). The 3-question Medication Satisfaction Questionnaire, completed by parents at the study endpoint, indicated that 97.1% was satisfied with LDX, while 87.3% reported they would absolutely or probably continue using LDX as their child's ADHD treatment. Overall, LDX appeared to have met parental expectations.
Parent Global Assessment at Endpoint (n=314).
NR2–051 Atilla Turgay, MD, University of Toronto, Toronto, Ontario, Canada
Improvement in executive function in children with attention deficit/hyperactivity disorder treated with 20 to 70 mg/d lisdexamfetamine dimesylate
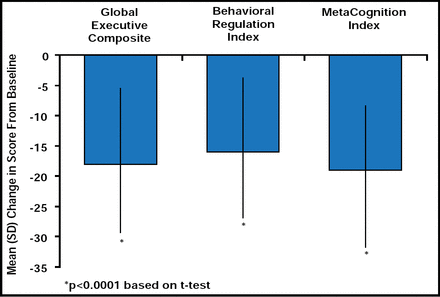
ADHD is often associated with deficits in executive function (EF) that play a role in goal-directed behavior, self-regulation, and control of emotional functioning [Barkley RA. Psychol Bull 1997; Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function (BRIEF): Professional Manual: Psychological Assessment Resources, Inc. 2000; Pennington BF, Ozonoff S. J Child Psychol Psychiatry 1996] and may contribute to the etiology of the disorder [Barkley RA. Psychol Bull 1997; Pennington BF, Ozonoff S. J Child Psychol Psychiatry 1996]. In an open-label, dose optimization trial, LDX significantly improved ADHD-RS-IV scores, measures of emotional expression, and EF after 7 weeks of daily treatment. At study end, changes in total EESC and subscale scores from baseline were: total score −7.4 ± 18.3; positive emotions subscale −2.1 ± 9.6; emotional flatness subscale −2.5 ± 7.7; and emotional lability subscale −2.8 ± 5.2. Subjects demonstrated significant (p<0.0001) improvement from baseline in all 8 components of the Behavior Rating Inventory of Executive Function (BRIEF)e scale and subscales. Global Executive Composite (GEC)e, Behavioral Regulation Index (BRI)e, and MetaCognition Index (MCI)e subscale scores at baseline and endpoint were similar across all LDX dose levels, while significant (p<0.0001) improvements in GEC, BRI, and MCI were noted at study endpoint (Figure 5). Outcome was not affected by baseline subtype of ADHD, previous psychiatric history, or the presence/absence of common TEAEs. After treatment with LDX, mean BRIEF total and subscale scores were below 65, a cutoff that is indicative of potential clinical significance.
Mean (SD) Brief T-Score From Baseline at End of Study (n=308).
NR2–052 Sharon B. Wigal, PhD, University of California, Irvine, CA
Sustained efficacy of lisdexamfetamine dimesylate over 13 hours as assessed by effect size in children with attention deficit/hyperactivity disorder
Drug effect sizes, calculated as the difference between drug effect and placebo effect divided by their pooled standard deviation (SD), can be used to estimate the efficacy of a drug across clinical trials [Faraone SV. Understanding effect size: how it's measured and what it means. 2008 cited; Available from: www.medscape.com/viewarticle/569729]. In this open-label, dose optimization study that was followed by a randomized, double-blind, placebo-controlled, 2-way cross study, the effect size of LDX was examined in an analog classroom setting. Subjects (n=129) who were administered LDX (30, 50, or 70 mg/day) had mostly medium to large effect size reductions in ADHD symptoms up to 13 hours postdose, as evaluated by a broad array of assessments, including clinician observations, classroom behavior, and academic performance. LDX significantly (p<0.05) improved SKAMP-Df compared with placebo at 1.5 hrs postdose. Mean treatment effect size over the treatment day was −1.73 ± 0.18. Mean raw postdose effect size was mostly large for SKAMP-Df, SKAMP-Af, SKAMP quality of workf, and SKAMPc total at all LDX doses and medium to large at most postdose time points. The effect size of LDX on PEMP-A and Cf was large and maintained over 13 hours. Overall effect sizes were robust, wherein the largest effect sizes were reported during the middle of the classroom day.

LDX adverse events profile in children aged 6 to 12 years
The adverse events (AEs) that were reported in the studies above were mild to moderate in severity, typical of other amphetamine AEs and consistent with other pediatric studies of LDX. Mild AEs were experienced by 42.6% of subjects, moderate by 40.1%, and severe by 2.2%. Treatment-emergent AEs that occurred in ≥10% of the study population were decreased appetite (43.2%), decreased weight (17.0%), insomnia (16.1%), irritability (16.1%), headache (13.9%), upper abdominal pain (13.2%), and initial insomnia (11.4%) [Biederman J et al. Clin Ther 2007]. Small changes in blood pressure and heart rate were noted at endpoint in 1 study (NR2–033).
Scales used in the studies above to assess ADHD and related functional deficits
aExpression and Emotion Scale for Children (EESC) is used to assess positive and negative emotions; subscales assess positive emotions, emotional flatness, and emotional lability [Perwien AR et al. J Atten Disord 2008].
bADHD Rating Scale IV (ADHD-RS-IV) and Clinical Global Impressions-Improvement (CGI-I) scores are used to evaluate hyperactivity and inattention [DePaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press 1998].
cThe Swanson, Kotkin, Agler, M-Flynn (SKAMP) scale assesses behavioral symptoms of ADHD in a classroom setting [Wigal SB, Gupta S, Guinta D, Swanson J. Psychopharmacol Bull 1998].
dParent Global Assessment (PGA) for improvement scale is a measurement tool that is used by the parent to assess changes in ADHD symptoms in his child.
eBehavior Rating Inventory of Executive Function (BRIEF) was designed to assess real-world executive function (EF) behaviors and comprises 86 items that are grouped into 8 scales that evaluate: inhibit, shift, emotion control, initiate, working memory, plan/organize, organization of materials, and monitor. The first 3 scales make up the Behavioral Regulation Index (BRI), and the last 5 make up the MetaCognition Index (MCI). Together, the BRI and MCI form the Global Executive Composite (GEC) score [Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function (BRIEF): Professional Manual: Psychological Assessment Resources, Inc. 2000].
fSKAMP Deportment (D) subscale evaluates a child's ability to interact with other children and adults, remain quiet, and staying seated in compliance with classroom rules. SCAMP Attention (A) subscale measures attention in the classroom. SCAMP quality of work subscale measures a child's ability to complete work accurately and neatly. Permanent Product Measure of Performance (PEMP) Attempted (A)/Correct (C) scale measures the number of problems attempted/number correct.
- © 2009 MD Conference Express