Summary
This article presents an overview of the prevalence, risk factors, mechanisms, treatments, and related clinical trials for smoking addiction.
- Smoking Cessation
- Substance-Related Disorders
- Prevention & Screening
Prevalence of Cigarette Smoking
Robert A. Kloner, MD, University of Southern California, Los Angeles, CA, presented an overview of the prevalence, risk factors, mechanisms, and treatments for smoking addiction during a special session at the American College of Cardiology Scientific Session in Orlando, Florida.
The World Health Organization estimates that there are 1.3 billion tobacco smokers globally, of which 47% are men and 12% are women [WHO Bulleitin 2004]. In Latin America and the Caribbean the Disease Control Priorities Project reports a rate of 40% for men and 24% for women [Disease Control Priorities in Developing Countries. Second edition. 2006, Table 46.1]. Although more than 70% of smokers want to quit and 30% to 50% attempt to quit each year, only 3% to 5% who succeed without some form of assistance remain cigarette free after 12 months [Hughes JR et al. Addiction 2004].3
Smoking as a Risk Factor Across an Array of Diseases
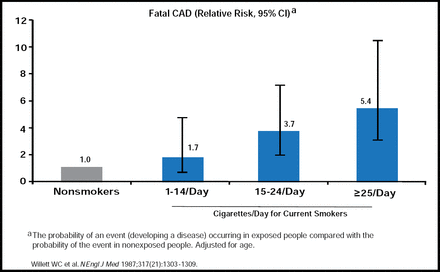
Tobacco use kills 5.4 million people a year-an average of one person every six seconds-and accounts for one in 10 adult deaths worldwide [http://www.who.int/topics/tobacco/facts.en]. It is the single largest cause of preventable death. The annual cost of health care directly related to smoking addiction is estimated at $96 billion [Mokdad AH et al. JAMA 2004]. The negative effects of smoking on the body are broad and varied. Smoking affects the cardiovascular, respiratory, and reproductive systems and is a leading cause of cancer in a number of different organs. In the cardiovascular system, smoking induces coronary artery disease (CAD) by reducing nitric oxide biosynthesis and increasing oxidative stress, white blood cell counts, thrombogenicity, and endothelial dysfunction. In addition, smoking is associated with a risk of angina, acute myocardial infarction (MI), sudden cardiac death, and Q-wave MI after percutaneous coronary revascularization. The risk of fatal CAD is directly correlated with the number of cigarettes consumed per day. Individuals smoking >25 cigarettes/day have a 5.4 greater risk of a fatal CAD event compared to nonsmokers (Figure 1)[Willett WC et al. N Engl J Med 1987]. Besides CAD, smoking is the cause of 12% to 14% of all stroke deaths [Goldstein LB et al. Stroke 2006].
Increased CAD Mortality.
Copyright © Massachusetts Medical Society 1987. All rights reserved.
Benefits of Smoking Cessation
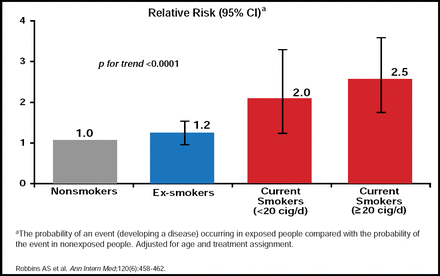
The benefits of ceasing to smoke are almost immediate, with cosmetic benefits noted within weeks and improved lung function seen after 3 months. After 1 year, the excess CAD risk attributable to smoking is reduced by 50% in ex-smokers, and after 15 years it is similar to that of individuals who had never smoked [CDC. MMWR 1990]. The OR for the risk of acute MI drops significantly from 2.95 (95% CI, 2.77 to 3.14) to 1.22 (95% CI, 1.09 to 1.37; p<0.0001) after 20 years of smoking cessation [Teo KK et al. Lancet 2006]. A similarly significant (p<0.0001) trend for a reduced risk of stroke is seen in ex-smokers (Figure 2)[Robbins AS et al. Ann Intern Med 1994].
Reduced Risk of Stroke.
Nature of Nicotine Addiction
The addictive characteristics of nicotine are a result of its rapid uptake and the subsequent intense release of dopamine in the brain. In addition, the α4β2 nicotinic acetylcholine receptors have a significant role in mediating the reinforcing and dependence-producing effects of nicotine [Coe JW et al. J. Med. Chem 2005]. Although smokers can self-regulate nicotine levels by selecting the frequency of cigarette consumption and choosing the intensity of inhalation [Schroeder SA. JAMA 2005], nicotine is rapidly cleared from the central nervous system. Its short half life (2 hours) in combination with tolerance-inducing up-regulation and decreased sensitivity of receptors can result in withdrawal symptoms including depressed mood, irritability, increased appetite, concentration difficulties, insomnia, anxiety and decreased heart rate [Han ES et al. Int J Clin Pract 2006]. Dr. Kloner said it was important to point out that tobacco dependence is a chronic medical condition and relapse is a component of this condition, not an indication of patient or clinician failure.
Pharmacotherapies as Aids to Smoking Cessation
As part of its Tobacco Free Initiative (TFI) the WHO recommends that treatment of tobacco dependence be addressed through a health systems approach that focuses on promoting and integrating clinical best practices (behavioral and pharmacological) which help tobacco-dependent consumers increase their chance of quitting successfully [http://www.who.int/tobacco/resources/publications/tobacco_dependence/en/index.html]. The American Association of Family Practice developed the ASK (only 70% of physicians ask patients about their tobacco use) and ACT (only 40% of physicians act to help patients with tobacco use dependency problems) programs. There are a number of counseling, as well as pharmacotherapy options (varenicline, bupropion HCl, and nicotine replacement therapy) to help smokers increase long-term smoking abstinence rates.
Robert M. Anthenelli, MD, University of Cincinnati College of Medicine, Cincinnati, OH, discussed the results from clinical trials of varenicline, the latest option for treating tobacco addiction. Varenicline functions as α4β2 nicotinic receptor partial agonist to inhibit dopaminergic activation produced by smoking, while simultaneously providing relief from the craving and the withdrawal syndrome that accompanies cessation attempts [COE JW et al. J Med Chem 2005]. This dual acting inhibitor blocks nicotine from binding to receptors and limits the release of dopamine, thus reducing the pleasure effect of nicotine and the reinforcing/rewarding effect of dopamine release.
Clinical Trials
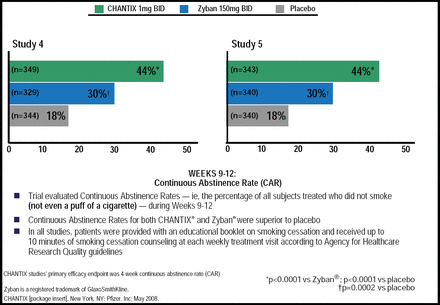
In two randomized clinical trials, varenicline (1 mg twice daily) was shown to reduce craving and withdrawal effects and, for those who smoked while receiving the study drug, smoking satisfaction. For Weeks 9 through 12, the 4-week continuous abstinence rates were 44.0% for varenicline versus 17.7% for placebo (OR, 3.85; 95% CI, 2.70 to 5.50; p<0.001) and versus 29.5% for bupropion SR (OR, 1.93; 95% CI, 1.40 to 2.68; p<0.001) for both trials. For weeks 9 through 52, the continuous abstinence rates were 21% and 22% for varenicline versus 8.4% and 10.3% for placebo (p<0.001) and 16.1% (p=0.057) and 14.6% (p=0.004) for bupropion SR (Figure 3)[Gonzales D et al. JAMA 2006; Jorenby DE et al. JAMA 2006]. The most common adverse events (AEs; >10%) for participants receiving varenicline were nausea (30%), insomnia (18%), headache (15%), and abnormal dreams (13%).
Varenicline Superior to Bupropion in 2 Head-to-Head Trials.
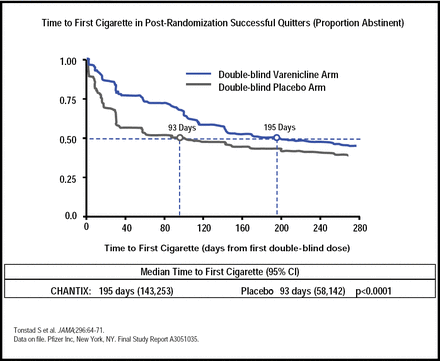
In another clinical trial to determine whether smokers who quit after 12 weeks of treatment with varenicline maintain higher abstinence rates than placebo-treated patients with an additional 12 weeks of treatment, abstinence rates for varenicline versus placebo subjects for Weeks 13 to 24 were 70.5% versus 49.6% (OR, 2.48; 95% CI, 1.95 to 3.16; p<0.001) and 43.6% versus 36.9% for weeks 13 to 52 (OR, 1.34; 95% CI, 1.06 to 1.69; p=0.02; Figure 4)[Tonstad S et al. JAMA 2006]. This data suggests that patients should receive additional treatment if they have early lapses after their target quit date.
Maintenance of Abstinence.
In patients with a history of MI, coronary revascularization, angina pectoris, peripheral arterial vascular disease, or cerebrovascular disease (CVD) diagnosed for >2 months, varenicline as a treatment for smoking cessation is efficacious and has an acceptable safety profile, according to a results of a study presented in a poster by Nancy A. Rigotti, MD, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
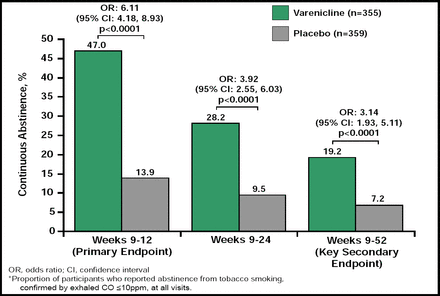
In this randomized, double-blind study, smokers (n=714) were randomly assigned to receive either varenicline (1.0 mg twice daily) or placebo for a 12 weeks. The target date for smoking cessation was 8 days after treatment initiation. Patients also received concurrent counseling. The 4-week continuous abstinence rate at the primary endpoint (Weeks 9 to 12) was significantly (OR, 6.11; 95% CI, 4.18 to 8.93; p<0.0001) higher for varenicline-treated subjects (47.0%) compared with placebo-treated subjects (13.9%; Figure 5). Abstinence rates for the varenicline-treated group were also significantly (p<0.0001) higher at Weeks 9 to 24 (28.2% vs 9.5%), and Weeks 9 to 52 (19.2% vs 7.2%). The 7-day point prevalence of abstinence rose over the 12 weeks of treatment for varenicline, and remained significantly (p<0.0001) elevated relative to placebo during and after drug treatment. The most frequent AEs in the varenicline group were nausea, headache, insomnia, vomiting and abnormal dreams. A total of 23 (6.5%) of varenicline-treated subjects experienced a serious AE compared to 21 (6.0%) of the placebo subjects. There were no differences in the number of adjudicated cardiovascular events (7.4% varenicline vs 6.6% placebo), cardiovascular deaths (0.3% vs 0.6%), or all-cause mortality (0.6% vs 1.4%). Though the authors cautioned that these results cannot be generalized for smokers with recent or acute CVD events, they concluded that varenicline has an acceptable safety profile with no evidence of increased risk of cardiovascular events or psychiatric side effects in smokers with stable CVD.
Continuous Tobacco Abstinence Rates.
- © 2009 MD Conference Express