Summary
Deep vein thrombosis (DVT) occurs in approximately 2 million Americans each year. Nearly 600,000 of these patients will develop a pulmonary embolism (PE), and almost 60,000 will die of this complication [Hirsh H & Hoak J. Circulation 1996]. The goal of antithrombotic therapy for venous thromboembolic (VTE) disease is the prevention of clot propagation, embolism, and recurrent thrombosis. This article reviews the 2008 ACCP evidence-based clinical practice guidelines regarding VTE disease, which established new strategies for the treatment of acute DVT and PE [Chest 2008;133 (6 suppl)].
- Thrombotic Disorders
- Thromboembolic Disease
Deep vein thrombosis (DVT) occurs in approximately 2 million Americans each year. Nearly 600,000 of these patients will develop a pulmonary embolism (PE), and almost 60,000 will die of this complication [Hirsh H & Hoak J. Circulation 1996]. The goal of antithrombotic therapy for venous thromboembolic (VTE) disease is the prevention of clot propagation, embolism, and recurrent thrombosis. John R. Bartholomew, MD, Cleveland Clinic, Cleveland, OH, reviewed the 2008 ACCP evidence-based clinical practice guidelines regarding VTE disease, which established new strategies for the treatment of acute DVT and PE [Chest 2008;133 (6 suppl)].
The 2008 guidelines now state that either subcutaneous unfractionated heparin (UFH) or fondaparinux is appropriate for the initial treatment of acute DVT and PE and can be administered on an outpatient basis without the need for constant monitoring.
For acute proximal DVT in select patients (symptoms for <14 days, good functional status, life expectancy >1 year, and low risk of bleeding), catheter-directed therapy is preferred to systemic thrombolysis. Thrombolytic therapy with fondaparinux is now indicated as a treatment for PE in selected high-risk patients who are hemodynamically stable and at a low risk of bleeding. Rapid risk stratification should be performed before beginning treatment.
For the first idiopathic DVT and PE events in patients without risk factors for bleeding, long-term anticoagulation is now recommended. Anticoagulation should be maintained for 3 to 6 months for secondary prevention of VTE and for more than 12 months for recurrent VTE. Low-intensity warfarin therapy (target INR, 1.5–2.0) is a highly effective method of preventing recurrent VTE [Ridker PM et al. N Engl J Med 2003] if the patient has a preference for less frequent INR testing.
John A. Heit, MD, Mayo Clinic, Rochester, MN, presented recent findings regarding the indications and diagnostic testing for thrombophilia, a hereditary or acquired coagulation disorder that may result in thrombosis. Approximately 5% to 8% of the US population has a genetic propensity for this disorder [Heit JA. Hematology 2007], which has been linked to both recurrent miscarriage and complications during pregnancy. Congenital conditions that increase the tendency to clot include deficiencies of antithrombin III, protein C or S, the presence of homozygous factor V Leiden or combined heterozygous factor V Leiden and prothrombin G20210A, and lupus anticoagulant/anticardiolipin antibodies. Temporary conditions that can result in hypercoagulability include the presence of antiphospholipid antibodies and pregnancy.
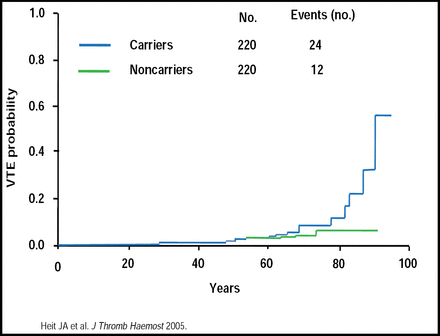
The relative risk for thrombosis varies. Older (≥60 years of age) Leiden carriers have an increased risk of VTE (Figure 1) [Heit JA et al. J Thromb Haemost 2005]. Activated protein C (APC) resistance is a frequent risk factor for symptomatic postoperative DVT and has an estimated relative risk of 5.0 (95% CI, 1.9 to 12.9) in elective replacement of the hip or knee [Lindahl TL et al. Thromb Haemost 1999]. A 50-fold increased risk was found in factor V Leiden carriers with a leg injury compared with noncarriers without injury (OR, 49.7; 95% CI, 6.8 to 362.7) [van Stralen KJ et al. Arch Intern Med 2008]. The overall risk of venous thrombosis was increased 7-fold in patients with a malignancy (OR, 6.7; 95% CI, 5.2 to 8.6) versus persons without malignancy.
Increased Risk of VTE in Older Leiden Carriers.
Hereditary thrombophilia is associated with occurrence at an early age, during pregnancy, family history, and idiopathic or recurrent VTE. Although a variety of tests, such as APC resistance, lupus anticoagulant testing, protein C and protein S activity, and factor V Leiden mutation assay, are used to diagnose thrombophilia, test reliability can be influenced by current anticoagulant use. Protein C and S deficiency testing should be avoided during warfarin therapy, and testing for lupus anticoagulant and antithrombin deficiency should be avoided during heparin therapy. Dr. Heit also recommends avoiding functional testing during acute thrombosis. Although DNA-based testing can be performed anytime, it may be inaccurate after bone marrow or liver transplant.
Management of thrombophilia in asymptomatic carriers and family members should include counseling regarding estrogen exposure, pregnancy, and other risk exposures. It has been suggested that the duration of primary prophylaxis should be increased for those with transient risk exposure (eg, surgery); however, this is not supported by clinical data.
“Thrombophilia can be difficult to diagnose and treat,” said Dr. Heit. “If you don't do this regularly, ask for help,” he cautioned.
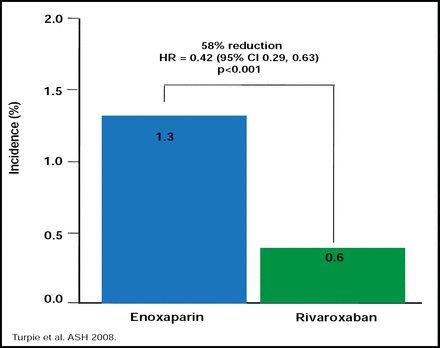
Despite the ACCP recommendations of thromboprophylaxis for patients who are admitted to the hospital with acute medical illness, anticoagulants are only administered to approximately 50% of patients who need them. Similarly, only 50% of patients who should receive warfarin for the prevention of stroke and systemic embolism in atrial fibrillation actually get it. Although warfarin remains the anticoagulant of choice in atrial fibrillation and is substantially more efficacious (by approximately 40%) than antiplatelet therapy [Hart RG et al. Arch Intern Med 2007], it is not without problems. Alexander G. G. Turpie, MD, McMaster University, Hamilton, ON, Canada, noted that several new anticoagulants that currently are in development, like dabigatran (IIa inhibitor), apixaban, and rivaroxaban (direct factor Xa inhibitors), are thought to have favorable pharmacokinetic characteristics that may overcome the limitations of warfarin. Studies with the new agents are ongoing across the spectrum of thromboembolic diseases, and the newer therapies are expected to be noninferior, if not more effective, than current anticoagulants, with similar or even less bleeding risk, according to Dr. Turpie (Figure 2).
Effectiveness of Enoxaparin vs Rivaroxaban.
Heparin-induced thrombocytopenia (HIT) is a potentially devastating complication of therapy with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). More than half of all patients who develop HIT will experience a thrombotic complication, most commonly DVT and PE, stated Teresa L. Carman, MD, University Hospitals Case Medical Center, Cleveland, OH. Typical HIT begins 4 to 14 days after heparin exposure, while rapid onset HIT is associated with a significant platelet drop within 24 hours of starting heparin. Delayed onset HIT can happen 9 to 40 days after discontinuation of heparin. In 1 series, 12/14 patients with delayed onset HIT were cardiac patients [Rice L et al. Ann Intern Med 2002].
“The cause of thrombosis while on anticoagulation may be difficult to sort out,” said Dr. Carman. Although it could be HIT, it also might be the result of prophylaxis failure or inadequate heparin administration/resistance. HIT-associated disseminated intravascular coagulation (DIC) also can be confounding. DIC is reported in 5% to 15% of HIT patients. It is associated with hypofibrinogenemia, elevated coagulation studies, and significant elevations in D-dimer. Awareness of HIT is crucial for the clinician, and sorting out the clinicopathological diagnosis from alternative causes for thrombocytopenia is necessary to make the diagnosis, cautioned Dr. Carman.
Platelets, once thought to be solely involved in clot formation, are now known to be key mediators in various others processes, such as inflammation, thrombosis, and atherosclerosis. Supported by the wealth of evidence from clinical trials that have demonstrated their benefits in patient outcomes, antiplatelet agents have become critical in the prevention and management of various diseases that involve the cardiovascular, cerebrovascular, and peripheral arterial systems. Deepak L. Bhatt, MD, VA Boston Healthcare System, Boston, MA, reviewed the outcomes for a number of antithrombotic therapy studies.
In the CURE study, clopidogrel had beneficial effects in patients with acute coronary syndromes (ACS) without ST-segment elevation. However, the risk of major bleeding is increased [Yusuf S et al. N Engl J Med 2001]. Clopidogrel pretreatment that is followed by long-term therapy in patients with ACS who receive aspirin was shown to be more beneficial than placebo in reducing major cardiovascular events in the PCI-CURE study [Mehta SR et al. Lancet 2001]. In the CREDO trial, 1-year treatment with clopidogrel significantly reduced the risk of adverse ischemic events in patients who received percutaneous coronary intervention (PCI) [Steinhubl SR et al. JAMA 2002]. The WRIST trial confirmed that clopidogrel treatment for 1 year was superior to a 6-month treatment course in reducing overall major cardiac events and revascularization rates, at least for patients with in-stent restenosis who were treated with gamma-radiation [Waksman R et al. Circulation 2002]. The benefits of dual antiplatelet therapy using clopidogrel+aspirin remain questionable but may be beneficial in patients with previous myocardial infarction or plaque rupture without any increased risk of severe bleeding in the long term [Bhatt DL et al. J Am Coll Cardiol 2007].
Mitul B. Kadakia, MD, Brigham and Women's Hospital, Boston, MA, presented the results of an analysis that examined the use of antithrombotics and their associated bleeding risk in patients with myocardial infarction. The analysis, based on data from 69,000 patients from the ACTION Registry, showed that overall unfractionated heparin (UFH) was the most commonly used agent, followed by low molecular weight heparin (LMWH), bivalirudin, LMWH + UFH, and no anticoagulant agent. LMWH was used more often in NSTEMI patients. When adjusted for baseline bleeding risk by the validated CRUSADE Bleeding Risk Score, patients with STEMI had higher rates of bleeding than those with NSTEMI.
Three posters that were presented by investigators from Brigham and Women's Hospital, Boston, MA, provided important information from a TRITON-TIMI-38 analysis about the use of prasugrel in subjects (n=13,608) with acute coronary syndrome (ACS) who were undergoing PCI.
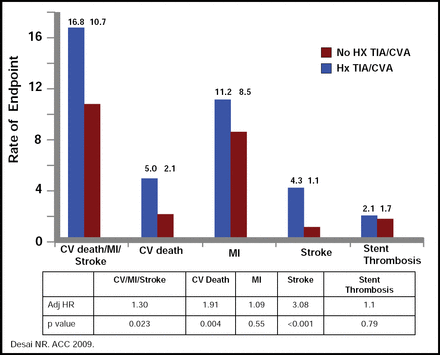
Data that were presented by Michelle O'Donoghue, MD, Brigham and Women's Hospital, Boston, MA, showed that the concomitant use of drugs that are metabolized by, or induce or inhibit, CYP 450 enzymes, such as PPIs, lipophilic statins, tobacco, and amiodarone, did not change the efficacy of prasugrel or the excess risk of TIMI bleeding. Further, the efficacy and safety of prasugrel are unaffected by the presence of near-complete inhibition of platelet aggregation with a glycoprotein IIb/IIIa inhibitor. Results of a subanalysis of the same data, however, showed prasugrel to be less effective in patients with a history of TIA or CVA. In presenting these data, Nihar R. Desai, MD, Brigham and Women's Hospital, Boston, MA, noted that this patient population had significantly higher rates of cardiovascular death, stroke, and bleeding (Figure 3). This suggestion of harm, said Dr. Desai, indicates that prasugrel should not be used in these patients.
Risk of CV, Death, Stroke and Stent Thrombosis.
- © 2009 MD Conference Express