Summary
Rosuvastatin did not improve cardiovascular morbidity and mortality in patients who had end-stage renal disease and who were on hemodialysis, according to results of the large, randomized, placebo-controlled A Study to Evaluate the Use of Rosuvastatin in Subjects On Regular hemodialysis: an Assessment of Survival and Cardiovascular Events [AURORA; NCT00240331] trial. There was no difference between rosuvastatin 10 mg and placebo in reducing the combined endpoint of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction or any of the individual components of this endpoint when analyzed separately.
- Cardiology Clinical Trials
- Lipid Disorders
- Renal Disease
Rosuvastatin did not improve cardiovascular morbidity and mortality in patients who had end-stage renal disease (ESRD) and who were on hemodialysis, according to results of the large, randomized, placebo-controlled AURORA (A study to evaluate the Use of Rosuvastatin in subjects On Regular hemodialysis: an Assessment of survival and cardiovascular events; NCT00240331) trial. There was no difference between rosuvastatin 10 mg and placebo in reducing the combined endpoint of cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction (MI) or any of the individual components of this endpoint when analyzed separately. The results were presented by Bengt Fellström, MD, University Hospital, Uppsala, Sweden [Fellström B et al. New Engl J Med 2009].
A number of studies have shown that statins lower the incidence of cardiovascular events in high-risk patients; however, it was unknown if they would have a similar effect in patients with ESRD who were on hemodialysis. A previous study with atorvastatin failed to demonstrate that statins have a statistically significant effect in reducing cardiovascular events in diabetic patients on hemodialysis with ESRD [Wanner C et al. New Engl J Med 2005].
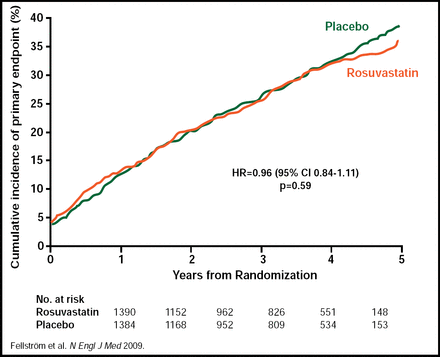
Results from AURORA showed no statistically significant difference between the rosuvastatin 10 mg daily group and the placebo group in the primary endpoint of major cardiovascular event (defined as cardiovascular death, nonfatal MI, or nonfatal stroke). A major cardiovascular event occurred in 396 rosuvastatin-treated subjects and 408 subjects who received placebo (HR, 0.96; 95% CI, 0.84 to 1.11; p=0.59; Figure 1). There were no statistically significant differences in any of the secondary endpoints, including any death (p=0.51), noncardiovascular death (p=0.34), major cardiovascular event-/cause-specific death (p=0.30), atherosclerotic cardiac event (p=0.64), vascular access procedure for hemodialysis (p=0.19), and coronary or peripheral revascularization (p=0.88). Rosuvastatin achieved a 43% reduction in LDL cholesterol at 3 months, from a mean baseline level of 100 mg/dL (2.6 mmol/L), compared with a 1.9% reduction in the placebo group (p<0.001). Rosuvastatin reduced total cholesterol at 3 months by 26.6% from baseline, compared with a 0.5% reduction in the placebo group (p<0.001), and triglyceride levels were reduced by 16.2% from baseline in the rosuvastatin group, compared with an increase of 0.9% in the placebo group (p<0.001). Adverse event rates were similar between the 2 groups. In the rosuvastatin group, 1140 (82.1%) subjects suffered a serious adverse event versus 1159 (84.1%) in the placebo group (p=0.80; Table 1). Drug-related adverse events occurred in 16 (1.2%) subjects in the rosuvastatin group and 11 (0.8%) subjects in the placebo group (p=0.35; Table 1). The baseline characteristics between the 2 groups were balanced. Mean duration of treatment was 2.4 years, and mean duration of follow-up was 3.2 years.
Adverse Events for Rosuvastatin 10 mg vs Placebo.
AURORA Primary Study Endpoint of Major Cardiovascular Event.
Copyright © Massachusetts Medical Society 2009. All rights reserved.
The AURORA study was an international, multicenter, randomized, double-blind, prospective trial and was the largest and longest trial ever conducted on the efficacy of statins on cardiovascular events in persons with ESRD on hemodialysis. Patients with ESRD have advanced calcification of the arteries, and the lack of benefit on CV outcomes that was observed with statin therapy in 4D and AURORA suggests that cardiovascular disease in patients who receive chronic hemodialysis differs from that in other clinical settings. Inclusion criteria were men and women aged 50 to 80 years who had ESRD and were on hemodialysis for at least 3 months. Major exclusion criteria were taking a statin within the previous 6 months and needing a kidney transplant within the following year. The study design required 2750 subjects, and the study continued until 620 subjects had a major cardiovascular event. Assessment was every 3 months for the first year and every 6 months thereafter until the study's conclusion.
During the question and answer session after the presentation, an audience member asked if the lack of efficacy resulted from the trial dose only being 10 mg. Dr. Fellström replied, “I wouldn't be surprised if it was a matter of dose.”
- © 2009 MD Conference Express