Summary
This article presents findings from another prespecified analysis of the JUPITER data, assessing the effect of rosuvastatin on symptomatic venous thromboembolism (VTE), which occurred about as often as myocardial infarction or stroke in the JUPITER study. Compared with placebo, rosuvastatin was associated with a 43% reduction (HR, 0.57; 95% CI, 0.37 to 0.86; p=0.007) in risk of VTE and no increase in bleeding [Glynn RJ et al. N Engl J Med 2009].
- Thrombotic Disorders
- Lipid Disorders Clinical Trials
A number of presentations highlighted new analyses from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER; NCT00239681) study, the results of which are expected to have a significant impact on the screening and treatment of cardiovascular disease (CVD). JUPITER was a primary preventive, prospective, randomized trial that included 17,802 men (aged ≥50 years) and women (aged ≥60 years) with no CVD or diabetes mellitus, and low-density lipoprotein (LDL) cholesterol and high-sensitivity C-reactive protein (hsCRP) levels <130 mg/dL and ≥2 mg/L, respectively. Subjects received either rosuvastatin (20 mg/day) or placebo. The trial was stopped prematurely after a median follow-up of 1.9 years due to clear and significant treatment benefits, wherein rosuvastatin produced a 44% reduction in the primary study endpoint (cumulative incidence rate of myocardial infarction [MI], stroke, arterial revascularization, hospitalization for unstable angina, or cardiovascular death) compared with placebo (HR, 0.56; 95% CI, 0.46 to 0.69; p<0.00001) [Ridker PM et al. N Engl J Med 2008].
Robert Glynn, PhD, Brigham and Women's Hospital, Boston, MA, presented findings from another prespecified analysis of the JUPITER data, assessing the effect of rosuvastatin on symptomatic venous thromboembolism (VTE), which occurred about as often as MI or stroke in the JUPITER study. Compared with placebo, rosuvastatin was associated with a 43% reduction (HR, 0.57; 95% CI, 0.37 to 0.86; p=0.007) in risk of VTE and no increase in bleeding [Glynn RJ et al. N Engl J Med 2009].
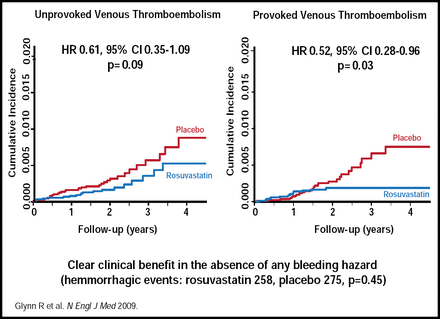
Rosuvastatin reduced the occurrence of both provoked (p=0.03) and unprovoked (p=0.09) VTE (Figure 1). Although the incidence of both pulmonary embolism and DVT was reduced, DVT alone was significantly reduced (p=0.004). The benefit of rosuvastatin was consistent across patient subgroups, based on baseline variables, while VTE reduction was independent of a prior cardiovascular event. Among patients who had VTE as the first event, there was a significant 43% reduction in risk (HR, 0.57; 95% CI, 0.37 to 0.86; p=0.007), similar to the 44% reduction in risk that was associated with rosuvastatin for the prevention of a first cardiovascular event.
JUPITER Venous Thromboembolism—Unprovoked versus Provoked.
Copyright © Massachusetts Medical Society 2009. All rights reserved.
When questioned regarding the likely underlying mechanisms of rosuvastatin, Dr. Glynn said he believed that the most likely candidate was an anticoagulant effect, noting that statins downregulate the blood coagulation cascade through decreased tissue factor expression, leading to reduced thrombin formation, as reported by Undas et al [Undas A et al. Arterioscler Thromb Vasc Biol 2005]. “Widening the treatment target to include prevention of VTE in addition to arterial thrombosis will increase the benefits of statin use,” Dr. Glynn concluded.
Paul Ridker, MD, Brigham and Women's Hospital, Boston, MA, discussed results of another prespecified subanalysis that compared clinical outcomes between JUPITER trial participants according to achieved levels of LDL and hsCRP. The findings established hsCRP as a biomarker of risk for cardiovascular disease not only in people with known risk factors but also in asymptomatic individuals who previously were considered at average or even low risk for MI, stroke, or death from cardiovascular causes [Ridker PM et al. Lancet 2009].
In this subanalysis (87% of full cohort), the clinical outcomes of JUPITER trial participants were evaluated according to achieved levels of LDL (≥70 or <70 mg/dL) and hsCRP (≥2 or <2 mg/L).
After adjusting for baseline variables, rosuvastatin-treated subjects who achieved a reduction in LDL levels to <70 mg/dL had a 55% reduction in cardiovascular events (HR, 0.45; 95% CI, 0.34 to 0.60; p<0.0001); those who achieved an hsCRP reduction <2 mg/L had a 62% reduction in event rate (HR, 0.38; 95% CI, 0.26 to 0.56; p<0.0001), and those who achieved both a reduction of LDL <70 mg/dL and hsCRP <2 mg/L had a 65% CV event reduction (HR, 0.35; 95% CI, 0.23 to 0.54; p<0.0001). In individuals who achieved an LDL reduction of <70 mg/dL and hsCRP reduction of <1 mg/L, there was a 79% event rate reduction (HR, 0.21; 95% CI, 0.09 to 0.52; p<0.001). Similar effects were observed in analyses that were based on apolipoprotein (Apo) B or ApoB: ApoA ratio rather than on LDL.
Dr. Ridker pointed out that the impact of hsCRP reduction appears to be independent of LDL, because less than 2% of the variance in achieved hsCRP was explained by the variance in achieved LDL. This fits with previous study results (PROVE IT-TIMI 22 and A to Z trials) that have indicated that in patients with acute coronary ischemia who were treated with statin therapy, greater clinical benefits were achieved when hsCRP levels were reduced to below 1 to 2 mg/L [Ridker PM et al. N Engl J Med 2005; Morrow DA et al. Circulation 2006].
Despite these encouraging results, Dr. Ridker stressed that for patients with raised LDL or raised hsCRP, initial interventions should include dietary restrictions, exercise, and smoking cessation. However, he estimated that applying the JUPITER screening and treatment strategy to the overall US population for 5 years could prevent more than 250,000 cardiovascular disease-related events.
- © 2009 MD Conference Express