Summary
This article discusses infection in cerebrovascular disease, including infection before and after acute ischemic stroke, infection and pediatric stroke, and virus vasculopathy among other things.
- Bacterial Infections
- Viral Infections
- Cerebrovascular Disease
- Inflammatory Diseases
Infection Before and After Acute Ischemic Stroke
“Extensive data now exist for a link between peripheral infection and stroke risk, especially acute bacterial infection,” said Hedley Emsley, MRCP, Royal Preston Hospital & University of Liverpool, Preston, UK.
A recent review of the literature revealed that the prevalence of infection in the week that precedes acute ischemic stroke (AIS) ranges between 10% to 35%, during which time infection confers a 2- to 3-fold increased stroke risk [Emsley HCA & Hopkins SJ. Lancet Neurol 2008]. This short window of elevated risk points to infection as a potential stroke trigger, with the highest risk period coinciding with the high-level proinflammatory phase of the immune response.
Poststroke infection complicates up to 30% of ischemic strokes, wherein both mortality (10% of deaths within 30 days of admission are due to pneumonia) and functional outcomes are adversely affected. The most consistently reported predictors of poststroke infection are advanced age, greater baseline stroke severity, total anterior circulation infarction, and dysphagia [Emsley HCA & Hopkins SJ. Lancet Neurol 2008].
“The presence of infection has several implications for clinical practice,” said Dr. Emsley. In terms of antecedent infection, vulnerable individuals may have a short-term increased stroke risk that is associated with acute systemic infection. Prevention is key; thus, adherence to vaccination schedules (eg, influenza) is important in vulnerable individuals. Evidence for poststroke “brain-induced” immunodepression derives from a range of studies. Most recently, results from the Preventive Antibacterial Therapy in Acute Ischemic Stroke (PANTHERIS) trial, suggested that markers of immunodepression are predictive of poststroke infection [Klehmet J et al. Neuroscience 2009].
Infectious Burden: A New Modifiable Risk Factor for Atherosclerosis?
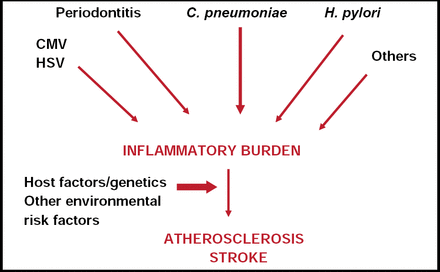
Atherosclerosis has a very large inflammatory component or could be considered an inflammatory condition. Mitchell Elkind, MD, Columbia University, New York, NY, said he believes that it is unlikely that there is one “stroke bug” that accounts for stroke. What is much more likely—if infection does play a role in atherosclerosis, stroke risk, and cardiac risk—is that several different infections may in sum contribute to an inflammatory burden that in turn contributes to atherosclerosis and potentially stroke. These mechanisms most likely also interact with host factors, genetic factors (in terms of ability to respond to infection), and perhaps other environmental factors as well (Figure 1).
“Burden” of Infectious Disease?
Dr. Elkind presented results from an ongoing population-based study of ischemic stroke incidence in an urban, multiethnic population (The Northern Manhattan Study) that used a quantitative weighted index to assess the risk of stroke associated with infectious burden. The results of this study lend support to the idea that past or chronic exposure to common infections, perhaps by exacerbating inflammation, contributes to atherosclerosis and stroke risk. Infectious burden also was associated with carotid plaque thickness and mortality. This study is limited by several factors: only 5 serologies were examined (C. pneumonia IgA, H. pylori IgG, CMV IgG, HSV 1 IgG, and HSV 2 IgG), there were no data on the virulence of any of the pathogens or the clinical history of the subjects, and stroke subtypes were not examined. In addition, the results showed only association, not causation.
“In the future,” said Dr. Elkind, “it will be important to look at additional pathogens (perhaps periodontal disease) and to include clinical data on the frequency and severity of infections as well as data on other subclinical measures of atherosclerosis.”
Infection and Pediatric Stroke
Heather J. Fullerton, MD, University of California, San Francisco, CA, discussed the results of 2 studies: the Kaiser Pediatric Stroke Study (KPSS), a retrospective cohort study that included 370 stroke cases, 97 of which were non-neonatal ischemic strokes; and the International Pediatric Stroke Study (IPSS), a multicenter, observational study that involved 1,187 cases of ischemic stroke, 676 of which were non-neonatal.
To assess infection as a risk factor for stroke, the KPSS investigators identified documented medical encounters for infection during the 4 weeks prior to stroke, or in the same time window in matched controls. Infection was divided into major (ie, sepsis, meningitis) and minor sources. Significantly more children with stroke had a medical encounter for infection in the month prior to their stroke versus controls (32% vs 8%; OR, 7.9; 95% CI, 3.7 to 16.8 for minor infections; 37% vs 8%; OR, 11.8; 95% CI, 5.2 to 26.8 for major + minor infections; both p<0.001). They also had significantly more medical encounters for infection than controls during the last 2 years.
Dr. Fullerton suggested 2 potential mechanisms for infection-related stroke: either the infection is inducing a systemic prothrombotic state or it is causing endothelial injury, leading to arteriopathy.
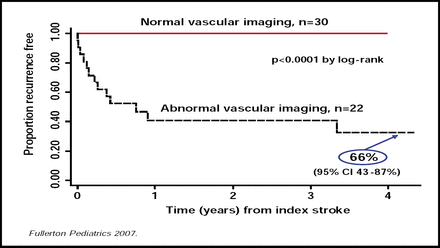
Recent data have indicated that arteriopathies are the major cause of stroke in otherwise healthy children who present with stroke [Ganeson VJ et al. Ann Neurol 2003]. Data from the KPSS study also show that arteriopathies are related to significant increases in stroke recurrence (5-year cumulative recurrence rate of 66%; 95% CI, 43 to 87; p<0.0001; Figure 2) [Fullerton HJ et al. Pediatrics 2007]. Idiopathic large vessel stenosis was the most common arteriopathy that was seen in both the KPSS and IPSS [LeFond A et al. Circulation in press] studies.
Arteriopathy is Significant and Increases the Risk of Recurrence of Stroke.
Fullerton Pediatrics 2007.
Although idiopathic arteriopathy may be due to trauma, several lines of evidence show that in cases in which there is evidence of progression in the first 2 to 6 months, it may be the result of infection, such as varicella. Other viruses that are associated with cerebral arteriopathies include herpes viruses, enterovirus, and HIV.
Dr. Fullerton said that additional studies of the vascular effects of infection in pediatric stroke are planned to measure associations between infection and stroke (case-control); infection and arteriopathy (cross-sectional); and infection/arteriopathy and recurrence (prospective cohort), with the long-term goal of developing secondary stroke prevention strategies in children.
The Protean Neurological Features of Varicella Zoster Virus Vasculopathy
Using a series of case studies, Donald H. Gilden, MD, University of Colorado School of Medicine, Denver, CO, discussed an updated description of varicella zoster virus (VZV) vasculopathies. He noted that they may be uni- or multifocal, that the resulting infarction is deep-seated more than superficial, and that white matter is involved more often than grey. Lesions are more often found in grey-white matter junctions and in both large and small arteries versus either large or small arteries alone. The spinal cord may become infarcted. Contrary to popular opinion, said Dr. Gilden, rash is found in only approximately 63% of patients and is not a requirement for diagnosis. Cerebrospinal fluid pleocytosis may include increased red blood cells but is absent in ∼30% of cases. Diagnostically, detection of VZV antibodies is superior to identifying VZV DNA. VZV vasculopathies can present with aneurysm, subarachnoid or cerebral hemorrhage, carotid dissection, or even peripheral arterial disease. In closing, Dr. Gilden said, “VZV is the only human virus proven to replicate in arteries.”
- © 2009 MD Conference Express