Summary
The most important treatment variable for a successful functional outcome following ischemic stroke is time to thrombolysis treatment. Recent evidence suggests that although earlier treatment leads to a better outcome, the treatment opportunity window is not fixed. This article discusses recent clinical trials that defined the optimal time window to maximize the effectiveness of reperfusion therapy in acute stroke.
- Cerebrovascular Disease
- Interventional Radiology
- Emergency Radiology
- Thrombotic Disorders
- Neuroimaging
The most important treatment variable for a successful functional outcome following ischemic stroke is time to thrombolysis treatment. Recent evidence suggests that although earlier treatment leads to a better outcome, the treatment opportunity window is not fixed. Joseph P. Broderick, MD, University of Cincinnati College of Medicine, Cincinnati, OH, presented recent clinical trials that defined the optimal time window to maximize the effectiveness of reperfusion therapy in acute stroke.
The odds of a favorable 3-month outcome on the modified Rankin Scale (mRS 0–2) have been shown to decrease as the onset time to treatment (OTT) decreases, wherein the best outcomes (OR, 2.8; 95% CI, 1.8 to 4.5) are associated with 0 to 90-minute window with recombinant tissue plasminogen activator (rt-PA) therapy [Hacke W et al. Lancet 2004]. The third European Cooperative Acute Stroke Study (ECASS-3) extended the treatment window to 3 to 4.5 hours. More patients had a favorable outcome with the plasminogen activator alteplase than with placebo [52.4% vs 45.2%; OR, 1.34; 95% CI, 1.02 to 1.76; p=0.04; Hacke W et al. N Engl J Med 2008]. Patients in the alteplase group had significantly (p<0.03) lower entry NIH Stroke Scale (NIHSS) scores and fewer prior strokes than the placebo group, which may have favored the active treatment group. A similar study, the Safe Implementation of Thrombolysis in Stroke—International Stroke Thrombolysis Register (SITS-ISTR) study, confirmed these results [Wahlgren N et al. Lancet 2008]. Variability and reversibility of focal ischemia are a function of a number of variables (eg, age, NIHSS score, stroke history) as well as variable collateral circulation, Dr. Broderick speculated, but speed of reperfusion is key.
Telemedicine, defined as real-time, 2-way audio and video, and digital imaging communication, may offer a means of getting the expertise of the shrinking supply of vascular neurologists to the staff of rural and community hospitals. David C. Hess, MD, Medical College of Georgia, Augusta, GA, discussed his recent work, which uses telemedicine to treat stroke patients at a distance. A recent study showed that telemedicine is superior to the telephone when making decisions to use tPA therapy [Meyer BC et al. Lancet Neurol 2008]. Correct treatment decision (98% of the time with telemedicine vs 82% with the telephone), high rates of thrombolysis use, improved data collection, low rate of intracerebral hemorrhage, low technical complications, and favorable time requirements all support the efficacy of telemedicine for making treatment decisions (Table 1). Dr. Hess described the REACH (Remote Evaluation of Acute Ischemic Stroke) Hub and Spoke Telestroke model for early treatment strategies as well as a means to enroll more patients into clinical trials. The web-based system allows stroke consultants to obtain histories, perform live video examinations, and review CTs online. A recommendation is made regarding the administration of rt-PA before patient transport to the tertiary medical center.
Telemedicine versus Telephone in Acute Stroke Patients.
Dr. Hess believes that telemedicine will enable the treatment and enrollment of a larger and more diverse population of patients in clinical trials and allow faster dissemination of clinical trial results throughout the real world.
The appearance of a larger lesion by perfusion-weighted magnetic resonance imaging (PWI) than by diffusion-weighted MRI (DWI) quantitatively defines a mismatch of potential clinical importance. Stephen M. Davis, MD, University of Melbourne, Melbourne, Australia, believes that this mismatch area can potentially identify subgroups of stroke patients who are likely to benefit from reperfusion in the 3- to 6-hour window. The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study showed that early reperfusion was associated with significantly increased odds of achieving a favorable clinical response in patients with a PWI/DWI mismatch (OR, 5.4; p=0.039) and an even more favorable response in patients with the target mismatch profile (OR, 8.7; p=0.011), while patients without a mismatch profile did not appear to benefit from early reperfusion [Albers G et al. Ann Neurol 2006].
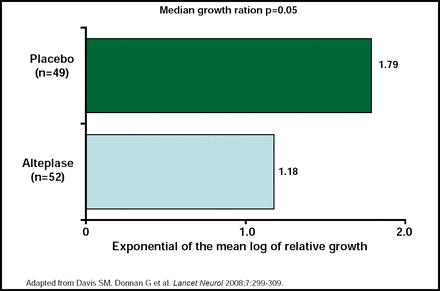
The Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) confirmed the DEFUSE findings. In patients who had a mismatch, reperfusion was more common with alteplase given 3 to 6 hours after stroke onset than with placebo and was associated with less infarct growth (p=0.05), better neurological outcome (p<0.0001), and better functional outcome (p=0.01) than no reperfusion (Figure 1; Davis SM et al. Lancet Neurol 2008). These results are contrary to the findings that were reported for the Desmoteplase In Acute Ischemic Stroke 2 (DIAS-2) trial [Hacke W et al. Lancet Neurol 2008], in which little clinical benefit and poor correlations with MRI mismatch after desmoteplase treatment, given 3 to 9 hours after the onset of stroke, were reported. However, Dr. Davis believes that MRI mismatch is the best validated penumbral strategy and may be improved by rapid online penumbral imaging.
EPITHET Trial—Primary Outcome (Infarct Growth).
Studies since the mid-1990s have consistently supported the use of hypothermia to protect the brain against ischemic damage following an acute stroke [Aronowski J et al. Stroke 1994]. James Grotta, MD, University of Texas, Houston, TX, presented preliminary results from the Intravascular Cooling in the Treatment of Stroke-Longer tPA window (ICTuS-L) study, which uses intravascular cooling (iced saline induction) plus rt-PA in the treatment of stroke. The target temperature of 33° to 34° C was reached in 1 to 3 hours in awake patients and maintained for 24 hours. Meperidine or buspirone was used to control shivering. Hypothermia was associated with a high incidence of pneumonia (48%), but there was no increase in deep vein thrombosis or hemorrhage. Endovascular cooling can be safely combined with thrombolytic therapy. A phase II/III study is planned with a target temperature of 33° at <6 hours from symptom onset.
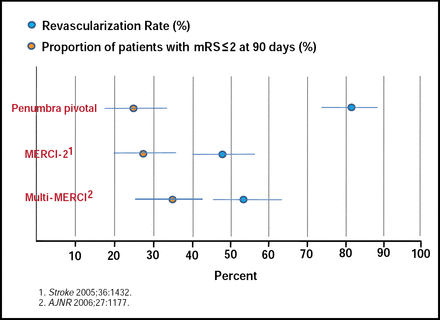
Wade Smith, MD, University of California, San Francisco, CA, reviewed the current trials that have employed intra-arterial (IA) lytics (PROACT-II) and mechanical devices (IMS-I, IMS-II, MERCI, Multi-MERCI, and Penumbra) for treating ischemic stroke. The PROACT-II (Prolyse in Acute Cerebral Thromboembolism II) trial is the only positive intra-arterial trial to date that has used recombinant prourokinase lytic (r-proUK) to treat a middle cerebral artery (MCA) occlusion. The recanalization rate was 66% for the r-proUK group and 18% for the control group (p<0.001) when therapy was started 3 to 6 hours poststroke [Furlan A et al. JAMA 1999]. The IMS (Interventional Management of Stroke) I and II trials examined the use of rt-PA that was administered via the EKOS® micro-infusion catheter. These trials showed that the reopening of occluded vessels beyond 400 minutes resulted in an outcome that was similar to those patients who did not recanalize. All trials that involved clot removal devices (MERCI-I, multi-MERCI, and Penumbra) reported high recanalization rates (60% to 82%; Figure 2). The question remains, however, whether rates of recanalization are associated with higher functional or mortality outcome rates. Three new trials are in the process [IMS-III, MR-RESCUE, and RETRIEVE] of attempting to answer this question.
Revascularization versus Functional Recovery Rate.
- © 2009 MD Conference Express