Summary
This article discusses the 180-day data from the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage [CLEAR; NCT00784134] study, which showed that the combination of extraventricular drainage plus thrombolysis with the thrombolytic rt-PA substantially reduces mortality and poor outcomes in patients with intraventricular hemorrhage.
- Neurology Clinical Trials
- Ischemia
- Interventional Techniques & Devices
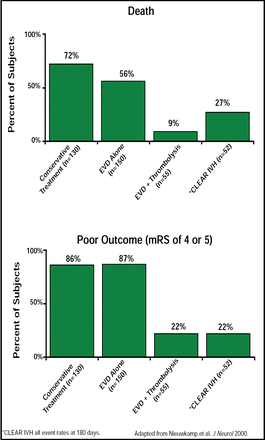
Daniel F. Hanley, MD, Johns Hopkins University, Baltimore, MD, reported 180-day data from the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR; NCT00784134) study, which showed that the combination of extraventricular drainage (EVD) plus thrombolysis with the thrombolytic rt-PA substantially reduces mortality and poor outcomes in patients with intraventricular hemorrhage (IVH). Severe IVH, as the result of subarachnoid hemorrhage or intracerebral hemorrhage, is difficult to treat and leads to hydrocephalus and frequent poor functional outcomes. A meta-analysis of 7 observational studies reported fatality rates of 9% (RR, 0.13; 95% CI, 0.04 to 0.40) for ICH/IVH patients who were treated with EVD plus fibrinolytic agents compared with 78% for conservative treatment and 58% for EVD alone. The authors concluded that such findings warranted a randomized clinical trial [Nieuwkamp et al. J Neurol 2000]. Based on these earlier studies, the CLEAR study (n=52) was designed to assess whether the addition of rt-PA to EVD improves functional outcome compared with EVD alone. Entry subjects included patients with IVH <30 cc and with a hematoma that was stabilized at 6 hours post-IVH. Intracranial catheters were placed in the frontal portion of the least involved lateral ventricle in about 85% of patients. In this dose escalation study, subjects were treated for 4 days or until the third and fourth ventricles opened and lateral shift was reduced. To assess the effect of drug delivery on clot lysis, computer tomography was used to assess the rate of clot lysis in all of the ventricles.
Safety and functional outcomes were measured at 30, 90, and 180 days post-IVH. Functional outcomes were assessed with the modified Rankin Scale (mRS), the Barthel score, NIH Stroke Scale (NIHSS), and Glasgow Outcome Score (GOS). No safety threshold was crossed in CLEAR rates. Mean patient age was 55 (10.3) years; baseline IVH volume was 30 to 40 cc; and ICH volume was 2.5 cc. The majority (19%) of the clots formed in the caudate region.
Mortality, symptomatic bleeding, and bacterial ventriculitis percentages were 17%, 4%, and 2% at 30 days, respectively. Functional outcomes at 180 days were as follows: 17 patients had mRS scores between 0–3 (0=no disability; 3=moderate disability); average Barthel score=93.8; average NIHSS=1.17 (0 indicates normal); and GOS=1.17 (1 indicated good recovery). Overall, these patients were highly functional as indicated by these scales. Those patients (n=13) who scored 0 to 3 on the mRS also demonstrated good daily functionality as measured by the Stroke Impact Scale (SIS-16), validating the mRS measurements. Five patients were totally normal. By 180 days, 27% (14/52) of the patients had died and 22% (11/15) were rated as having poor outcomes (mRS of 4 or 5). The effect of rt-PA on good clinical outcome at 30 days (mRS 0–4) had an OR of 0.24 (Figure 1).
CLEAR IVH All Event Rates at 180 Days.
The positive findings from this study will allow the authors to proceed to a CLEAR Phase III trial, which has a projected enrollment of 500 patients at 50 to 70 worldwide sites that have neurosurgical and stroke expertise. The hypothesis that is to be tested in this placebo-controlled, blinded, randomized trial is whether EVD plus rt-PA treatment of IVH obstruction in the third and fourth ventricles will increase the percentage of patients in the mRS 0 to 3 group compared with EVD alone.
- © 2009 MD Conference Express