Summary
Thiazolidinediones, such as rosiglitazone, have been shown to increase insulin sensitivity and reduce other cardiovascular risk factors but increase fluid retention and the risk of heart failure. This article discusses results of the Assessment on the Prevention of Progression by Rosiglitazone on Atherosclerosis in Diabetes Patients with Cardiovascular History [APPROACH; NCT00116831] trial.
- diabetes mellitus
- lipid disorders
- prevention & screening clinical trials
- prevention & screening
Results of the APPROACH trial (Assessment on the Prevention of Progression by Rosiglitazone on Atherosclerosis in Diabetes Patients with Cardiovascular History; NCT00116831) were presented by Richard W. Nesto, MD, Lahey Clinic, Burlington, MA, at the American Heart Association Scientific Sessions meeting in New Orleans.
Thiazolidinediones, such as rosiglitazone, have been shown to increase insulin sensitivity and reduce other cardiovascular (CV) risk factors but increase fluid retention and the risk of heart failure. It has been hypothesized that they also may reduce the progression of coronary atherosclerosis, although prior studies on CV outcomes have been mixed. The objective of the APPROACH trial was to assess the effect of the thiazolidinedione rosiglitazone versus the sulfonylurea glipizide on intravascular ultrasonography (IVUS) measures of atherosclerosis in native coronary arteries.
APPROACH was a multinational, double-blind, randomized, controlled trial that was conducted among subjects with type 2 diabetes and a clinical indication for angiography or percutaneous coronary intervention. Patients who had ≥1 nonintervened plaques with a 10% to 50% narrowing of the coronary artery were eligible for participation. The primary study endpoint was percent change in atheroma volume (PAV) from baseline to 18 months using IVUS, as analyzed by a blinded core laboratory. Secondary endpoints included changes in normalized total atheroma volume and atheroma volume of the most diseased 10-mm coronary artery segment.
Subjects were randomly assigned to receive rosiglitazone that was titrated to 8 mg/day (n=233) or glipizide that was titrated to 15 mg/day for 18 months (n=229). Metformin or insulin could be added after 3 months as needed to attain a target hemoglobin A1c (HbA1c) ≤7%. Other CV risk factors were managed according to regional guidelines and clinical judgment.
A total of 672 subjects enrolled in the study; 462 had both a baseline and 18-month IVUS. Study subjects had a mean age of 61 years (32% women), and a median of 4.8 years passed since their diabetes had been diagnosed. Eighteen percent of subjects were not on medication for their diabetes, 54% was on 1 medication, and 28% was on dual therapy. Patient characteristics were similar between both treatment groups except for blood pressure, which was higher in the glipizide group (131/76 vs 128/75 mm Hg), and creatinine, which was slightly higher in rosiglitazone subjects (1.02 vs 0.98 mg/dL; both p<0.05).
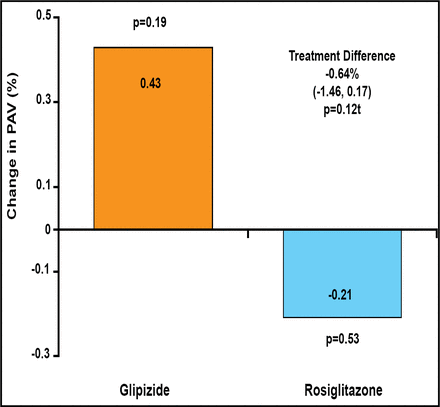
After 18 months, neither treatment produced a significant difference in the primary endpoint, PAV change (rosiglitazone −0.21%; p=0.53; glipizide +0.43%; p=0.19). There was no significant difference between treatment groups (−0.64%; 95% CI, −1.46 to 0.17; p=0.12; Figure 1).
APPROACH Primary Endpoint – Change in PAV.
Rosiglitazone produced a significant change in normalized total atheroma volume (−3.9 mm3; p<0.05). The change also was significant when compared with glipizide (−5.12 mm3; 95% CI, −9.98 to −0.26; p=0.04). Both treatments produced a significant change in atheroma volume in the most diseased 10-mm coronary artery segment (−5.3 mm3 and −3.6 mm3, rosiglitazone and glipizide, respectively; both p<0.0001); the difference between treatment groups was not significant (−1.7 mm3; 95% CI, −3.93 to 0.49; p=0.13). The achieved reductions in HbA1c were similar (−0.2% for glipizide vs −0.3% for rosiglitazone; p=0.44).
There were no significant differences in major CV events. Hypoglycemia was more frequent with glipizide (96 subjects vs 27 subjects for rosiglitazone; p<0.0001). Weight gain (2.6 kg vs 1.4 kg vs baseline; p=0.02) and a >3 g/dL decrease in hemoglobin (8% of subjects vs 3% of subjects; p=0.01) were more frequent with rosiglitazone.
In conclusion, in the APPROACH trial, 18-month treatment with rosiglitazone did not significantly reduce plaque volume in diabetics who had nonobstructive coronary plaques compared with glipizide, but secondary findings suggest that rosiglitazone may have a greater antiatherosclerotic effect than glipizide.
- © 2009 MD Conference Express