Summary
Following closely behind vildagliptin and sitagliptin, the two currently marketed compounds in the DPP-IV inhibitor drug class, are the pipeline agents saxagliptin and alogliptin. This article discusses data concerning all four molecules.
- endocrinology
- hyperglycemia/hypoglycemia
- diabetes mellitus
Following closely behind vildagliptin and sitagliptin, the two currently marketed compounds in the DPP-IV inhibitor drug class, are the pipeline agents saxagliptin and alogliptin; data for all 4 were presented in a single oral presentation.
Vildagliptin
The prevalence of kidney disease in patients with diabetes is reported to be between 20% and 40% (Parving. Diabetologia 1998). In order to insure the clinical safety of vildagliptin in patients with renal impairment, a pooled analysis was conducted by Tom Thuren, MD, PhD, and colleagues at Novartis Pharmaceuticals Corporation, East Hanover, NJ, and Novartis Pharma AG, Basel, Switzerland.
Safety data were pooled from 12 randomized trials of vildagliptin monotherapy in treatment-naïve type 2 diabetes mellitus (T2DM) patients and 8 studies in which vildagliptin was given as an add-on to prior monotherapy with other oral therapies or insulin. Efficacy data were pooled from 6 vildagliptin monotherapy trials. The entire dataset represents nearly 12,000 patients who were included regardless of renal status, and, with the exception of renal status, their baseline characteristics were similar.
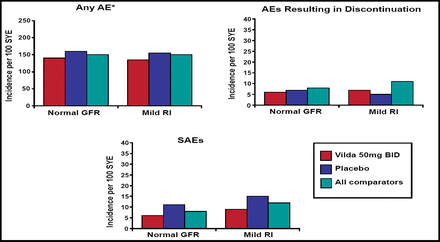
Results from the safety data show that at baseline, 6063 patients were receiving vildagliptin 50 mg qd or bid, and 1945 of them were defined as having renal impairment (≤80 mL/min/1.73m2). An analysis of this subpopulation did not detect any statistical difference in the rate of adverse events (AEs) between patients who were using vildagliptin and any of the comparator agents (Figure 1). For patients with normal and mildly impaired renal function, the incidence of AEs was similar for vildagliptin qd or bid, as well as pooled comparators and placebo. The improved HbA1c levels that were achieved with vildagliptin were equivalent regardless of renal status.
Incidence of Any AE, Any SAE, or Premature Discontinuation Due to an AE (Safety Pool).
Dr. Thuren concluded that the clinical safety and efficacy profile of vildagliptin were unaffected when it was used in patients with mildly impaired renal function but cautioned against vildagliptin use in the setting of moderate or severe renal impairment until more data become available.
Sitagliptin
D. Williams-Herman, MD, Merck Research Laboratories in Rahway, NJ, presented data for sitagliptin in combination with metformin as initial T2DM treatment from a 54- week study, with an extension arm that was followed out to 2 years.
Patients were randomized to one of 6 treatment arms:
-
sitagliptin (SITA) 100 mg qd
-
metformin (MET) 500 mg bid
-
MET 1000 mg bid
-
SITA 50 mg/MET 500 mg bid
-
SITA 50 mg bid/MET 1000 mg bid
-
Placebo (PBO)/MET 100 bid
One thousand ninety-one patients were randomized, with 511 completing the trial. The highest number of noncompleters was observed in the PBO and SITA 100 mg qd arms.
In the initial phase of the study, the reduction in HbA1c was observed to be the greatest in the SITA 50 mg/MET 1000 mg patient cohort (n=124).
Four hundred fifty-four patients in the extension study were eligible for analysis at 104 weeks (the highest retention rate was seen in the SITA 50 mg/MET 500 mg cohort). At baseline, patients in the extension phase had a median age of 54 years, a body mass index (BMI) of 31.1, and a median HbA1c of 8.5%.
In this long-term analysis, the greatest change in HbA1c was observed in the SITA 50 mg/MET 1000 mg cohort, with a reduction of 1.7% versus 1.4% for SITA 50 mg/MET 50 mg. The proportion of patients who were able to attain HbA1c targets of <7.5% at Week 104 was 60% for SITA 50mg/MET 1000mg and 45% for each of the SITA 50 mg/MET 1000 mg and MET 1000 mg treatment groups.
Body weight changes were highest for MET monotherapy, with slight weight gains (0.1–0.5 kg) observed in the SITA monotherapy and SITA 50 mg/MET 500 mg cohorts. AEs were similar among the active treatment groups. The rate of hypoglycemia was reported to be highest in the SITA 50 mg/MET 1000 mg cohort (5%) but was similar to all active treatment arms.
Saxagliptin
Initial antihyperglycemic monotherapy frequently is insufficient in enabling patients to reach their glycemic targets. Data that were presented by R. Chen, MD, Bristol-Myers Squibb, Princeton, NJ, suggest that saxagliptin (SAXA), given in combination with metformin as initial therapy, is superior to either agent used alone in treatment-naïve T2DM patients.
A 24-week study to examine the efficacy of saxagliptin, given in combination with metformin, the standard first-line therapy approach in T2DM, as initial therapy enrolled 1306 randomized and treated patients with inadequate glycemic control. Baseline patient characteristics included a mean HbA1c of 9.5%; BMI = 30 kg/m2; and duration of type 2 diabetes of 1.7 years. Patients were randomized to: PBO + MET; SAXA 10 mg + PBO; SAXA 5 mg + MET; or SAXA 10 mg + MET. In the 3 treatment arms that contained metformin, the metformin dose could be titrated to a maximum of 2000 mg/day in divided doses.
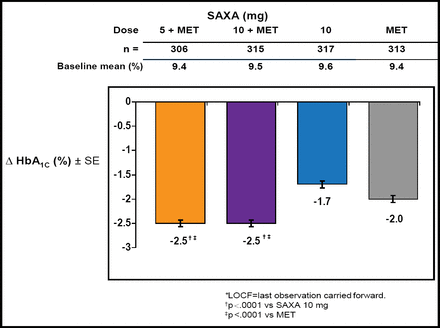
Results of this investigation showed that the HbA1c adjusted mean change from baseline to 24 weeks was −2.5% for the combination therapies – statistically superior to SAXA or MET monotherapy (p<0.0001; Figure 2). Those with higher HbA1c levels at baseline achieved the greatest drops in HbA1c (SAXA 5 mg + MET—baseline HbA1c ≥10%, −3.3%; baseline HbA1c <8%, −1.1%). There also was a statistically significant improvement for patients who reached their therapeutic target of <7% HbA1c in the combination arms as compared with monotherapy (p<0.0001).
HbA1c Adjusted Mean Change from Baseline at Week 24.
The frequency of AEs was comparable across active treatment groups. Overall, the most frequent events were nasopharyngitis, headache, diarrhea, and hypertension. Only 3 subjects were reported to have instances of confirmed hypoglycemia; 2 for the SAXA 10 mg + MET combination, and 1 for MET monotherapy. Similar degrees of weight loss from baseline were observed across all groups.
Alogliptin
Alogliptin (ALO) has been studied in 5 phase 3 trials as monotherapy or in combination with metformin, sulfonylureas, pioglitazone, and insulin. The present study, reported by P. Fleck, MT, Takeda Pharmaceuticals, Deerfield, IL, explored the use of alogliptin in combination with insulin.
In a double-blind, placebo-controlled study of 3 patient cohorts, patients received ALO 12.5 mg qd (n=131), ALO 25 mg (n=129), or PBO (n=129). In all cohorts, patients received insulin with or without metformin. The study endpoint was HbA1c levels at 26 weeks.
The resultant dataset includes data from 390 randomized subjects. At baseline, patients were an average age of 55 years; had a BMI = 32 kg/m2; had been diagnosed with T2DM for a median of 12 years; and were on a daily insulin dose of 56 U and a daily metformin dose of 1700 mg. The majority of the subjects (55% to 60%) was receiving insulin in combination with metformin prior to randomization; the remaining subjects were receiving insulin alone.
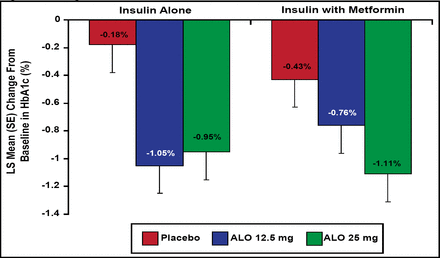
Results showed a significant change from baseline HbA1c at 26 weeks of −0.63% (ALO 12.5 mg) and −0.71% (ALO 25 mg) versus −0.13% for PBO (p<0.001). In addition, patients who were being treated with insulin alone or insulin with metformin were evaluated in a subgroup analysis. This analysis demonstrated that when ALO 25 mg was added to insulin in combination with metformin, subjects achieved the greatest HbA1c reduction of −1.11% from baseline (Figure 3). Significant decreases in fasting plasma glucose was observed only in the ALO 25 mg group compared with PBO (p<0.05). No difference in body weight changes was observed between ALO 12.5 mg and ALO 25 mg compared with PBO following 26 weeks of treatment.
Change From Baseline in HbA1c at Week 26.
AEs were similar for all 3 treatment arms, with the most common events being urinary tract infection, diarrhea, nasopharyngitis, and headache. There was no increase in the incidence of hypoglycemia when comparing ALO 12.5 mg and ALO 25 mg with PBO.
- © 2008 MD Conference Express