Summary
Treatment with an angiotensin-II receptor blocker may slow the development of retinopathy among patients with type 1 diabetes, according to findings from the Diabetic Retinopathy Candesartan Trials [DIRECT] Study Programme. Although the primary endpoint was not met, secondary findings suggest that inhibition of the renin-angiotensin system may lessen the risk of microvascular complications in this patient population.
- endocrinology
- retinal diseases
- diabetes & endocrinology clinical trials
- diabetes mellitus
Treatment with an angiotensin-II receptor blocker (ARB) may slow the development of retinopathy among patients with type 1 diabetes, according to findings from the Diabetic Retinopathy Candesartan Trials (DIRECT) Study Programme. Although the primary endpoint was not met, secondary findings suggest that inhibition of the renin-angiotensin system (RAS) may lessen the risk of microvascular complications in this patient population.
The DIRECT Programme involves 3 randomized controlled studies that enrolled a total of 5231 patients with type 1 or type 2 diabetes mellitus. The DIRECT-Protect 1 and DIRECT-Protect 2 trials were designed to evaluate the effect of candesartan on the progression of retinopathy in patients with type 1 and type 2 diabetes mellitus, respectively.
Nishi Chaturvedi, MD, Imperial College, London, UK, presented findings from the DIRECT-Prevent 1 trial, which was designed to evaluate the effect of candesartan on the incidence of new-onset retinopathy in type 1 diabetes. In DIRECT-Prevent 1, patients with type 1 diabetes who had no existing eye disease were randomly assigned to treatment with candesartan (n=710) or placebo (n=710) for at least 4 years.
Patients (mean age, 30 years) had an average duration of diabetes of 6.7 years at study entry. All patients had normal blood pressure (mean, 116/72 mm Hg) and kidney function (mean urinary albumin excretion rate, 4.5 μg/min).
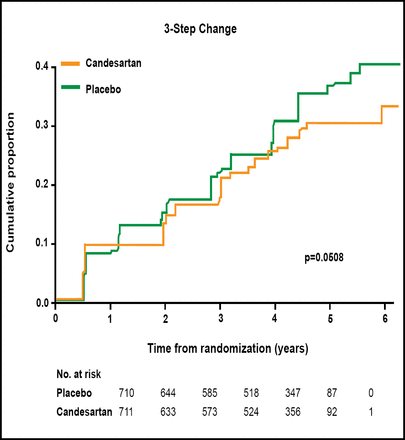
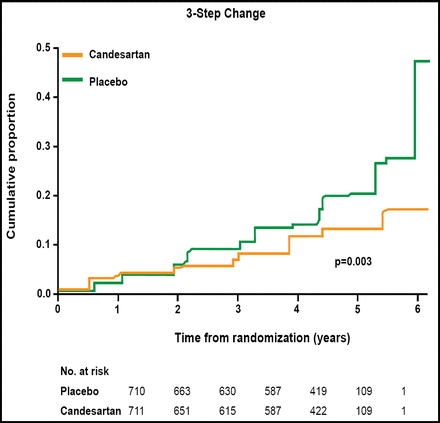
The primary endpoint was incidence of new retinopathy, as measured by 2-step change on the Early Treatment of Diabetic Retinopathy Study (ETDRS) scale. Candesartan reduced the incidence of diabetic retinopathy by 18% compared with placebo (p=0.0508; Figure 1). In a post hoc analysis that used more stringent criteria for the onset of eye disease (a 3-step change on the ETDRS scale), candesartan reduced the risk of retinopathy by 35% compared with placebo (p=0.003; Figure 2).
2-Step Change in Retinopathy Incidence.
3-Step Change in Retinopathy Incidence.
Adjustments for baseline blood pressure levels slightly lessened the magnitude of the treatment effect but did not change the overall findings. This suggests that the effects of candesartan on the development of diabetic retinopathy extend beyond the known blood pressure effects of RAS blockade, Prof. Chaturvedi said.
Treatment with candesartan was well tolerated in the DIRECT-Prevent 1 trial. The majority (80%) of patients in the candesartan arm received a daily dose of 32 mg for 4 to 6 years. The most common adverse events (AEs) among all patients were nasopharyngitis, hypoglycemia, hypotension, and headache. A similar proportion of patients in the candesartan and placebo groups experienced any AE (71.1% vs 72.8%) or discontinued study medication due to AEs (3.1% vs 2.5%).
“The take-home message is angiotensin inhibitors are indicated in patients with risk of progression into retinopathy,” said Kristian F. Hanssen, MD, PhD, Aker University Hospital, Oslo, Norway. Data from the DIRECT Programme and other trials should be used to develop an algorithm to help clinicians identify retinopathy in patients with type 1 or type 2 diabetes.
- © 2008 MD Conference Express