Summary
Results of the Dutch Echographic Cardiac Risk Evaluation Applying Stress Echo III [DECREASE III; ISRCTN83738615] study showed that extended-release fluvastatin therapy in addition to beta-blockers is associated with significantly improved perioperative cardiac outcomes in statin-naïve high-risk patients who undergo elective vascular surgery.
- imaging modalities
- interventional techniques & devices clinical trials
- cardiac imaging techniques
- lipid disorders
Results of the DECREASE III (Dutch Echographic Cardiac Risk Evaluation Applying Stress Echo III; ISRCTN83738615) study showed that extended-release fluvastatin therapy in addition to beta-blockers is associated with significantly improved perioperative cardiac outcomes in statin-naïve high-risk patients who undergo elective vascular surgery.
Patients who undergo noncardiac vascular surgery are at increased risk for coronary ischemic complications that have a perioperative cardiovascular mortality rate of approximately 2%. Recent retrospective and small prospective studies in patients who have been scheduled for vascular surgery suggest a potentially beneficial role for statins in the prevention of cardiac complications, in part as a result of their pleiotropic, anti-inflammatory effect. DECREASE III was a randomized, double-blind, placebo-controlled study that was conducted to assess the cardioprotective effect of sustained-release fluvastatin 80 mg in addition to beta-blocker therapy in vascular surgery patients. The study was conducted between June 2004 and April 2008 at the Erasmus Medical Center, Rotterdam, The Netherlands.
Men and women aged 41 years and older who were scheduled for elective noncardiac vascular surgery (ie, abdominal aortic aneurysm repair, abdominal aortic stenosis surgery, lower limb arterial reconstruction, or carotid artery stenosis repair) with moderate-high risk (>2% risk of perioperative cardiac death) were eligible to participate. Major exclusion criteria were current use of or contraindication for statin therapy, surgery that would interfere with continuous 12-lead ECG recording, unstable coronary artery disease (CAD), extensive stress-induced myocardial ischemia that was suggestive of left main disease, previous study participation, or reoperation within 30 days of an initial surgical procedure.
The primary outcome measure of the study was the occurrence of myocardial ischemia through 30 days, as assessed by continuous ECG monitoring and serial or troponin T measurement in the first 72 postoperative hours, and 12-lead ECG through the end of follow-up. The secondary efficacy endpoint was the composite of cardiovascular death and nonfatal myocardial infarction (MI) within 30 days after surgery. Safety endpoints were elevations in creatine kinase (CK) and AST/ALT, myopathy, and rhabdomyolysis.
Subjects (median age 65.7 years, 75% men, 39% prior CAD, 29% prior stroke, 20% diabetic) were randomly assigned to receive either extended-release fluvastatin (80 mg daily; n=250) or matching placebo (n=247), beginning 30 days prior to surgery and continuing for at least 30 days postsurgery. CK, ALT, and AST were measured 1, 3, 7, and 30 days after surgery. Fluvastatin was withheld if ALT levels exceeded 3X upper limit of normal (ULN), if CK levels exceeded 10X the (ULN), or if subjects experienced myopathy or rhabdomyolysis.
Treatment with fluvastatin reduced the baseline total cholesterol and LDL by 20% and 21%, respectively, compared with 4% and 3% reductions with placebo (p<0.001 for both). Similarly, fluvastatin reduced the inflammatory markers of high-sensitivity c-reactive protein and interleukin-6 by 21% and 33%, respectively, compared with changes of +3% and −4% with placebo (p<0.001 for both). Changes in HDL (+4% vs +2%) and triglycerides (+1% vs 0%) were similar between the treatment groups (both p=NS).
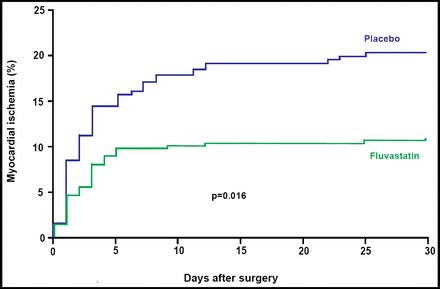
Significantly fewer subjects who received fluvastatin reached the primary study endpoint of myocardial ischemia within 30 days of the initial vascular surgical procedure. A total of 27/250 [10.9%] fluvastatin subjects were diagnosed with myocardial ischemia versus 47/247 [18.9%] placebo patients (OR 0.53; 95% CI, 0.32 to 0.88; p=0.016; Figure 1). The number that was needed to treat (NNT) to prevent one patient from experiencing myocardial ischemia was 13.
Primary Endpoint of Myocardial Ischemia Within 30 Days of the Initial Vascular Surgical Procedure.
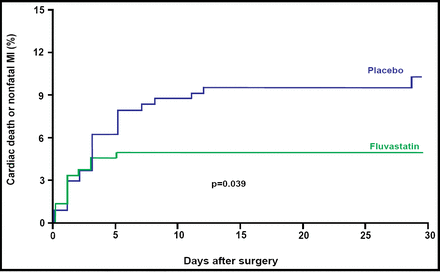
A total of 18 patients (3.6%) died within 30 days after surgery. Of these deaths, 12 (2.4%) were attributable to cardiovascular causes. Twenty-five patients (5.0%) experienced a nonfatal MI during that same period. Fluvastatin therapy was associated with a 52% relative risk reduction in the incidence of the combined secondary endpoint of cardiovascular death or MI (OR 0.48; 95% CI, 0.24 to 0.95; p=0.039;Figure 2). The NNT for the secondary composite endpoint of cardiovascular death or nonfatal MI was 19.
Secondary Endpoint of Cardiovascular Death or MI Within 30 Days After Surgery.
The proportion of patients who experienced any adverse event was similar between the fluvastatin and placebo groups. The percentage of patients who experienced a CK rise >10X the ULN was 4.1% in the fluvastatin group versus 3.0% in the placebo group (p=0.8). The percentage of patients who had a significant increase (>3X the ULN) in ALT levels was 3.1% in the fluvastatin group and 5.2% in the placebo group (p=0.3). Similar rates of study drug discontinuation were observed between the groups, and there were no cases of myopathy or rhabdomyolysis.
Commenting on the results of this study, Marc E. Shelton, MD, Prairie Heart Institute, Springfield, IL, expressed the opinion that extended-release fluvastatin should be considered for statin-naïve patients who undergo major peripheral vascular procedures and that, in light of the safety profile of fluvastatin, future studies of other statins should consider avoiding a placebo arm.
Of note, many of the patients who were enrolled in this study met current indications for aggressive lipid-lowering therapy due to the presence of prior CAD, stroke, or peripheral vascular disease, for whom the target LDL was <2.5 mM/L (optional target < 2.0 mM/L). While the study excluded patients who were on statins at the time of randomization, it is not clear how much of the benefit of extended-release fluvastatin that was observed in the trial was derived from those patients who should have been taking a statin prior to randomization. Nonetheless, the results of this trial are consistent with prior studies that have observed a beneficial effect of perioperative statin therapy in patients who are undergoing noncardiac surgery.
- © 2008 MD Conference Express