Summary
In a prespecified analysis of the TRITON-TIMI 38 trial, prasugrel was more effective and as safe as standard dose clopidogrel in patients with ST-elevation myocardial infarction (STEMI) who were undergoing planned percutaneous coronary intervention. This article presents an important analysis of patients with STEMI, a stratified group of patients at the time of randomization in the TRITON-TIMI 38 clinical trial.
- myocardial infarction
In a prespecified analysis of the TRITON-TIMI 38 trial, prasugrel was more effective and as safe as standard dose clopidogrel in patients with ST-elevation myocardial infarction (STEMI) who were undergoing planned percutaneous coronary intervention (PCI).
Gilles Montalescot, MD, PhD, Institut de Cardiologie, Pitie-Salpetriere Hospital, Paris, France, presented an important analysis of patients with STEMI, a stratified group of patients at the time of randomization in the TRITON-TIMI 38 clinical trial. The primary findings of the main trial, published in the New England Journal of Medicine [Wiviott et al. NEJM 2007], were that prasugrel decreased the composite of cardiovascular death, MI, or stroke by 19% [95% CI, 10% to 27%; p<0.001] compared with clopidogrel in patients with acute coronary syndromes who were undergoing planned PCI. The main study also found that prasugrel increased the rate of major hemorrhage (2.4% vs 1.8%; p=0.03), including life-threatening bleeding (1.4% vs 0.9%; p=0.01). These findings of increased efficacy and bleeding are consistent with the previously demonstrated faster achievement and higher degree of platelet inhibition that occur with prasugrel compared with clopidogrel.
In the TRITON-TIMI 38 STEMI analysis, 3532 patients who presented with STEMI were eligible for the trial if they underwent primary PCI within 12 hours of symptom onset (n=2438) or underwent secondary PCI within 14 days after initial medical therapy of STEMI (n=1094). This represented one of the largest randomized studies in mechanical reperfusion of STEMI, the largest experience with prasugrel for PCI in STEMI, and the first comparative study of clopidogrel in patients with STEMI who were undergoing PCI.
Patients were randomized to prasugrel (60 mg load, 10 mg QD maintenance) or clopidogrel (300 mg load, 75 mg QD maintenance), with the first dose administered within 1 hour of leaving the catheterization laboratory and the dose continued for a median duration of 15.2 months. PCI was performed in 97% of patients, wherein 92% received an intracoronary stent (59% bare metal stent only, 33% drug-eluting stent).
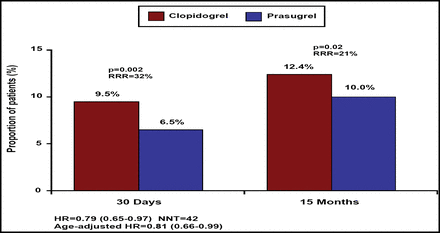
Cumulative Incidence of Primary Study Endpoint (Cardiovascular Death, Nonfatal MI, Nonfatal Stroke) in the Whole STEMI Population, for the First 30 Days.
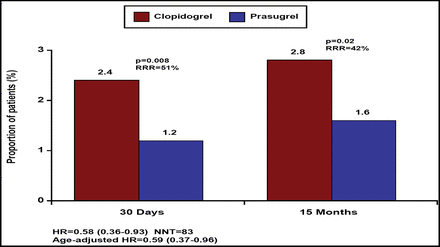
The primary endpoint (composite of cardiovascular death, MI, or stroke through 15 months) was reduced by 21% (relative) in patients who were randomized to prasugrel (12.4% vs 10.0%; p=0.02; Figure 1). Similarly, the key secondary endpoint of cardiovascular death, MI, or urgent target vessel revascularization through 30 days also was reduced by 25% in the prasugrel group (8.8% vs 6.7%; p=0.02). Furthermore, stent thrombosis (definite or probable stent thrombosis according to the Academic Research Consortium definition) was reduced by 51% (2.4% vs 1.2%; p=0.008) at 30 days and by 42% through the end of follow-up (2.8% vs 1.6%; p=0.02; Figure 2).
Cumulative Incidence of Stent Thrombosis in the Whole STEMI Population (ARC Definite or Probable Stent Thrombosis).
At 15 months, there was no increase in the primary safety endpoint of TIMI major non-CABG bleeding between prasugrel (2.4%) and clopidogrel (2.1%; p=0.65). Likewise, life-threatening non-CABG bleeding (1.3% vs 1.1%; p=0.75) and TIMI major or minor non-CABG bleeding (5.1% vs 4.7%; p=0.65) were similar between treatment groups.
Prof. Montalescot presented results using two definitions of net clinical benefit at 15 months – the first was a composite of death, MI, stroke, or major non-CABG bleeding, and the second was a composite of death, MI, stroke, or all major bleeding. Using both definitions, prasugrel achieved a more favorable net clinical benefit (14.6% vs 12.2%; p=0.02 first definition; 14.7% vs 12.5%; p=0.04 second definition) compared with clopidogrel.
In conclusion, patients with STEMI who were undergoing planned PCI in the TRITON-TIMI 38 trial experienced superior reduction in ischemic events with prasugrel compared with clopidogrel, without an increase in bleeding. Stent thrombosis in the first 30 days was reduced by >50% with prasugrel. These data strongly support a role for prasugrel as an alternative to clopidogrel in patients with STEMI who are undergoing primary or secondary PCI.
- © 2008 MD Conference Express