Summary
The goals for the management of schizophrenia and schizoaffective disorders are to decrease severity and frequency of psychosis and associated mood symptoms while reducing the inherent risks of antipsychotic medications. Aripiprazole demonstrates agonist activity toward dopamine D2 and 5-HT1a receptors and antagonizes 5-HT2a receptors, sites that are associated with psychosis and mood disorders.
- psychiatry clinical trials
- schizophrenia
- psychopharmacology
The goals for the management of schizophrenia and schizoaffective disorders are to decrease severity and frequency of psychosis and associated mood symptoms while reducing the inherent risks of antipsychotic medications. Aripiprazole demonstrates agonist activity toward dopamine D2 and 5-HT1a receptors and antagonizes 5-HT2a receptors, sites that are associated with psychosis and mood disorders. Its moderate-to-lack of affinity for histamine H1 and cholinergic muscarinic receptors suggests a favorable safety profile.
Pooled data from two 52-week efficacy studies that compared aripiprazole (20–30 mg/day) with haloperidol (7–10 mg/day) in patients with early (aged =40 years with first episode and duration of illness =60 months) schizophrenia [Crandall D et al. NR4–028] showed that significantly (p=0.001) more of the haloperidol (29%) patients discontinued treatment versus aripiprazole-treated patients (11%). Remission rates, defined by the Remission in Schizophrenia Working Group, were significantly higher with aripiprazole (38% vs 22%; p=0.003) and were achieved in a shorter time versus haloperidol.
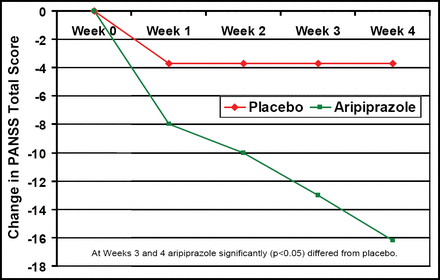
In another study that compared aripiprazole treatment (mean 23.9 ± 6.4 mg/day) with placebo in patients with schizoaffective disorder, Glick ID et al. (NR4–039) reported fewer discontinuations with aripiprazole (42%) versus placebo (57%). PANSS total score was significantly (p<0.05) improved after 3–4 weeks of aripiprazole treatment versus placebo (Figure 1). Adverse events were similar and included anxiety, vomiting, dizziness, akathisia, tremor, and depression. Although EPS symptoms and metabolic values were similar, prolactin levels decreased more in the aripiprazole group (−5.6 vs −1.3; p<0.0001).
Mean Change from Baseline to Endpoint in the PANSS Total Score.
At Weeks 3 and 4, aripiprazole differed significantly (p<0.05) from placebo
In a pooled analysis of data from 5 studies, Kane JM et al. (NR4–097) noted significant decreases in PANSS total score after 4 weeks of treatment with aripiprazole (5–30 mg/day) versus placebo (−14.4 vs −2.4; p=0.001). The largest effect was noted for the hostility and uncooperativeness symptom domains.
In a further analysis of the above data and symptom domains, Janicak P et al. (NR4–080) reported that negative, positive, depression/anxiety, disorganized thought, and hostility domains significantly (p<0.05) improved after aripiprazole treatment versus placebo beginning at Week 2 in patients who were diagnosed as schizophrenic or having schizoaffective disorder.
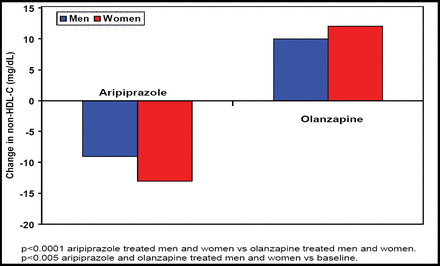
Meyer JM et al. [NR4–053] noted that non-HDL cholesterol, a strong predictor of cardiovascular disease (often associated with schizophrenia treatment), significantly (p<0.001) decreased after aripiprazole treatment versus significant (p<0.001) increases after olanzapine treatment (Figure 2).
Mean Change in Non-HDL at Week 26.
In general, the investigators concluded that aripiprazole appears to be an effective first-line treatment for schizophrenia symptoms that has a favorable adverse event profile.
- © 2008 MD Conference Express