Summary
Over 50% of schizophrenia patients drop out of controlled trials within 6 months due to lack of efficacy or in some cases tolerability issues [Liu-Siefert et al. BMC Med 2005; Lieberman JA et al. N Engl J Med 2005], particularly extrapyramidal symptoms (EPS), akathisia, weight gain, metabolic effects, sedation, and anticholinergic effects. EPS and akathisia effects carry an increased risk of suicide [Dong JY et al. J Affect Disord 2005] and poor adherence, while metabolic effects can increase the risk for diabetes and cardiovascular disease. Iloperidone, a mixed D2/5-HT2 antagonist that is under US Federal Drug Administration (FDA) review, may provide a lower EPS/akathisia liability.
- psychiatry clinical trials
- psychopharmacology
- schizophrenia
Over 50% of schizophrenia patients drop out of controlled trials within 6 months due to lack of efficacy or in some cases tolerability issues [Liu-Siefert et al. BMC Med 2005; Lieberman JA et al. N Engl J Med 2005], particularly extrapyramidal symptoms (EPS), akathisia, weight gain, metabolic effects, sedation, and anticholinergic effects. EPS and akathisia effects carry an increased risk of suicide [Dong JY et al. J Affect Disord 2005] and poor adherence, while metabolic effects can increase the risk for diabetes and cardiovascular disease. Iloperidone, a mixed D2/5-HT2 antagonist that is under FDA review, may provide a lower EPS/akathisia liability.
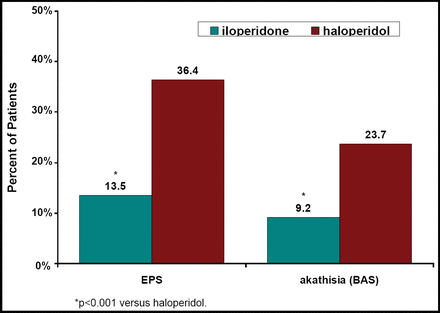
A combined analysis of three 52-week studies that compared the efficacy of iloperidone (4–16 mg/day) versus haloperidol (5–20 mg/day) in schizophrenia patients showed that 36.4% of patients in each treatment group had discontinued [Torres R et al. NR4–093]. Relapse occurred in 43.5% of the iloperidone and 41.2% of the haloperidol patients. Improvement in the CGI-C scores occurred in 65% of iloperidone and 66% of haloperidol patients. Iloperidone was non-inferior to haloperidol [HR 1.03, 95% CI 0.74, 1.43; p=0.86]. Safety results from this study [Wolfgang CD et al. NR4–024] showed that 4.3% of iloperidone patients versus 8.5% of haloperidol patients discontinued because of an adverse event. Fewer iloperidone patients experienced worsening of their EPS or akathisia scores versus haloperidol (Figure 1).
Patients (%) With Worsening EPS and Akathisia From Baseline to Endpoint.
Efficacy results of a 28-day phase 3 trial [Weiden PJ et al. NR4–078] showed significant decreases in PANSS scores for patients who were treated with iloperidone 12 mg BID (−12.0; p=0.006) or ziprasidone 80 mg BID (−12.3; p=0.012) versus placebo (−7.3). Safety results [Hamilton J et al. NR4–046] showed discontinuation rates of 35%, 34%, and 40% for iloperidone, ziprasidone, and placebo, respectively. Adverse events that were associated with iloperidone included dizziness, sedation, and modest weight increase versus sedation, dizziness, and akathisia for ziprasidone. A significantly (p<0.05) higher percentage of ziprasidone patients experienced worsening of EPS scores and akathisia versus placebo, a finding that was not noted with iloperidone.
Pooled data from 9 phase 2 and 3 clinical trials showed that iloperidone, haloperidol, ziprasidone, and risperidone produced similar weight gains in patients [Stahl SM et al. NR4–102]. The percentage of patients who had a ≥7% change in body weight was 23.2% for iloperidone, 17.6% haloperidol, 14.2% risperidone, and 7.4% ziprasidone (short-term study only). Iloperidone demonstrated favorable metabolic effects on glucose, cholesterol, and triglycerides, and unlike risperidone and haloperidol, it was not associated with hyperprolactinemia. Lauriello J et al. (NR4–050) reported that iloperidone use had fewer incidences of EPS and akathisia versus the other antipsychotics that were tested.
In general, the researchers in these studies concluded that iloperidone was as effective as the existing antipsychotic treatments for schizophrenia but may be associated with a lower incidence of EPS/akathisia.
- © 2008 MD Conference Express