Summary
Over 60% of patients with major depressive disorder (MDD) who are treated with standard antidepressant therapy (ADT) do not achieve remission. The efficacy and safety of aripiprazole as adjunctive therapy recently have been demonstrated in patients who had an inadequate response to initial ADT [Berman RM et al. J Clin Psychiatry 2007; Marcus RN et al. J Clin Psychopharmacol 2008]. This article discusses posters, presented during the American Psychiatric Association 2008 Annual Meeting, which reported additional analysis of data from the above studies.
- mood disorders
- psychopharmacology clinical trials
Over 60% of patients with major depressive disorder (MDD) who are treated with standard antidepressant therapy (ADT) do not achieve remission. The efficacy and safety of aripiprazole as adjunctive therapy recently have been demonstrated in patients who had an inadequate response to initial ADT [Berman RM et al. J Clin Psychiatry 2007; Marcus RN et al. J Clin Psychopharmacol 2008].
Posters that were presented during the APA annual meeting reported additional analysis of data from the above studies, which were identical 14-week trials that consisted of an 8-week prospective treatment phase with ADT and a 6-week randomized, placebo-controlled phase in which patients received either adjunct aripiprazole (n=375; mean dose 11.1 mg/day) or adjunct placebo (n=368). Only patients who failed to achieve an adequate response (<50% reduction on HAM-D17 Total, HAM17 ≥ 14, and CGI-I ≥3) with ADT entered the randomized phase.
Carlson BX et al. (NR3–014) reported that patients who were treated with adjunct aripiprazole for 6 weeks experienced a 1.75-kg increase in weight versus 0.38 kg in placebo patients (p<0.001). Common treatment-emergent adverse events that were seen more often in aripiprazole versus placebo patients included akathisia (28.8%), restlessness (12.1%), insomnia (8.1%), fatigue (8.4%), blurred vision (5.7%), and constipation (4.6%). The majority of akathisia events that were experienced was mild/moderate and rarely led to discontinuation; 52% resolved by the study end and 80% resolved following a dose reduction.
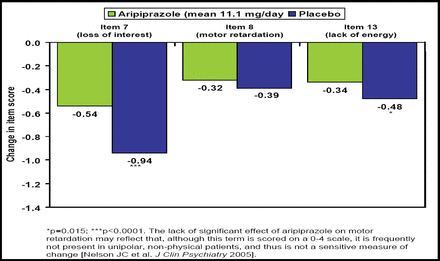
In an analysis of difficult-to-treat core symptoms of depression, Nelson JC et al. (NR3–022) reported that adjunctive aripiprazole produced significantly greater improvement in loss of interest (p=0.0001) and lack of energy (p=0.015) but not motor retardation (Figure 1). Overall composite score for these items, as well as HAM-D17 total score, was significantly improved with aripiprazole versus placebo (−1.61 vs −1.12; p <0.001 and −7.1 vs −4.7; p<0.001, respectively).
Reimherr FW et al. (NR3–039) reported that aripiprazole significantly (p<0.001) improved 8 of 10 MADRS line items, including apparent sadness, reported sadness, lassitude, and inability to feel, as early as 1 week after beginning treatment; pessimistic and suicidal thoughts improved within 2 weeks. Reduced sleep and appetite improved gradually with aripiprazole and reached significance (p<0.001) by Week 6.
Mean Change from Baseline (End of Week 8) to Endpoint (Week 14) in HAM-D Composite Drive Score Items.
For a subset of patients who were defined as anxious or having atypical depression, Trivedi MH et al. (NR3–074) reported that aripiprazole produced significantly (p<0.05) greater improvement in MADRS total score versus placebo. Response and remission rates also were significantly (p<0.05) higher in the aripiprazole treatment group.
Tran QV et al. (NR3–097) presented data that showed that the mean reduction in MADRS total score was significantly greater in patients with MDD who received adjunctive aripiprazole versus placebo (−8.67 vs −5.73; p<0.001). A subpopulation analysis revealed no treatment-by-subgroup interaction.
Overall, adjunctive aripiprazole appears to be an efficacious, safe, and well-tolerated treatment for the core symptoms of depression in patients who are resistant to ADT.
- © 2008 MD Conference Express