Summary
Despite the significant breakthroughs over the years for the treatment of rheumatic diseases, many autoimmune diseases remain resistant to treatment, or response is incomplete. In a basic science session, novel treatments from the fields of stem cells, gene therapy, and therapeutic vaccines are described.
- rheumatological autoimmune disorders
Despite the significant breakthroughs over the years for the treatment of rheumatic diseases, many autoimmune diseases remain resistant to treatment, or response is incomplete. In a basic science session, novel treatments from the fields of stem cells, gene therapy, and therapeutic vaccines were described.
Alan Tyndall, MD, University of Basel, Basel, Switzerland, discussed breakthroughs in the promising field of hematopoietic and mesenchymal stem cell research. He limited his talk to adult stem cells, which he defined as having the ability to divide and create another cell like itself and also to divide and create a cell that is more differentiated than itself. Adult stem cell research is less controversial, as the production of adult stem cells does not require the destruction of an embryo. There is some exciting research that suggests that it may be possible to reprogram an adult stem cell back to the embryonic stage, which offers the advantages of having unlimited expansion and pluripotency (having the potential to differentiate into endoderm, mesoderm, or ectoderm cell types), which would make it more like embryonic cells.
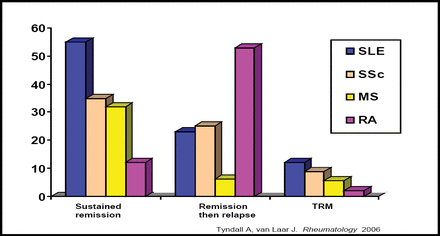
Hematopoietic stem cells have been used for over 30 years in the treatment of leukemia. Hematopoietic stem cell transplantation (HSCT), also known as bone marrow transplantation, is a special form of therapy that involves taking cells that normally are found in the bone marrow (stem cells) from a donor or from umbilical cord blood and reinfusing the cells into the recipient. The aim of this is to replace the normal hematopoietic system that has been eliminated by chemotherapy, along with the target cells, usually a malignancy such as leukemia. Results from animal models and coincidental cases of autoimmune disease in patients who received HSCT for malignant disease were encouraging enough to begin using HSCT for the treatment of severe autoimmune disease alone. Since 1996, over 900 patients who suffer from severe autoimmune disease have been treated with HSCT. Sustained remission was obtained in over 50% of SLE and 30% of systemic sclerosis (SSc) and multiple sclerosis patients (Figure 1). Rheumatoid arthritis (RA) patients had a low remission and high relapse rate and are no longer being considered for this therapy. Immune reconstitution data suggest a ‘resetting’ of autoimmunity in those patients who achieved stable remission rather than simply prolonged immunosuppression. Autologous HSCT, when given to carefully selected patients with severe diffuse cutaneous SSc, can produce a sustained improvement of skin thickening and stabilization of organ function up to 7 years after transplantation [Vonk MC et al. Ann Rheum Dis 2008]. This suggests that HSCT can reduce collagen and encourage small vessel remodeling.
A number of animal model studies have shown that mesenchymal stem cell transplantation has immunosuppressive, “homing” (homes to distressed tissue), and reparative properties. Mesenchymal stem cells have a marked proliferative capacity in vitro that is useful in multilineage differentiation for tissue engineering. Bone marrow-derived mesenchymal stromal cells also suppress the proliferation of stimulated lymphocytes by up to 90%. In addition, mature human articular chondrocytes, cells that have a mesenchymal origin, have marked antiproliferative effects on anti-CD3-stimulated lymphocytes [Bocelli-Tyndall C et al. Rheumatology 2006].
These encouraging results have inspired a number of worldwide clinical trials that are using stem cell therapy for the treatment of inflammatory autoimmune diseases.
Sustained Remission in Patients Treated with HSCT.
Gene Therapy for Arthritis
Paul-Peter Tak, MD, PhD, Academic Medical Center/University of Amsterdam and Arthrogen BV, Amsterdam, The Netherlands, discussed novel gene therapies for the treatment of autoimmune diseases in preclinical development. Intra-articular genes that are delivered by way of a recombinant adeno-associated virus (rAAV) that encodes anti-inflammatory proteins may be a safe and feasible approach for the treatment of RA. Of the serotypes that have been tested so far, AAV-5 appears to be the most efficient way to transfer intra-articular genes [Adriaansen J et al. Ann Rheum Dis 2005]. Treatment with the cytokine interferon (IFN)-β has been shown to reduce secretion of TNFα, IL-1β, and IL-6 (all key players in the pathogenesis of RA), inflammation, and synovial hyperplasia; inhibit arthritis progression; and protect against bone destruction in various animal models of RA [Tak PP. Front Biosci 2004]. However, clinical improvement could not be induced using systemic treatment with recombinant IFN-β in RA patients when administered only 3 times weekly, most likely due to pharmacokinetic issues. It appears likely that continuous levels of IFN-β at the site of inflammation are required to induce clinical efficacy, which might be achieved by intra-articular gene therapy After successful proof-of-concept studies, Dr. Tak has investigated the potential of intra-articular IFN-β gene therapy using an adenoviral vector and in follow-up studies of rAAV5 that expresses rat IFN-β, using different animal models of arthritis. Local delivery of vectors that express rat IFN-β after the onset of disease reduced paw swelling impressively in both the treated and untreated contralateral joints [Adriaansen J et al. Hum Gene Ther 2006; Adriaansen J et al. J Gen Virol 2007]. Strikingly, IFN-β gene therapy protected against joint destruction, which is a hallmark of RA. Dr. Tak believes that these results provide a strong rationale for the development of IFN-β gene therapy as a novel therapeutic approach for arthritis.
Therapeutic Vaccines
The target of therapeutic vaccines in immunotherapy is restoring immune tolerance while maintaining the response to exogenous antigens. New strategies that use active immunotherapy that is based on the vaccination principle are replacing passive immunotherapeutic approaches. Active immunotherapy either induces antibodies to a given antigen or induces T cells, mostly cytotoxic T lymphocytes, to neutralize the interaction of the self-cytokine to its receptor.
Marie-Christophe Boissier, MD, PhD, Institut National de la Santé et de la Recherche Médicale, Bobigny, France, outlined new strategies for the use of vaccination as immunotherapy.
Inhibition of TNFα, a primary cytokine that is involved in driving the inflammation process, with soluble TNFα receptors (etanercept) and TNF-binding monoclonal antibodies (infliximab, adalimumab, golimumab, certolizumab) combined with, chiefly, methotrexate has produced promising treatment outcomes. However, low long-term remission rates have generated interest in alternative strategies, such as modifying the TNFα molecule to contain foreign immunodominant T helper epitopes; administering naked DNA that encodes TNFα; linking the cytokine to a foreign carrier protein to circumvent Th cell tolerance; and using a peptide of the cytokine that is coupled to a macromolecule. Another discussed strategy is vaccination against proinflammatory cytokines (IL-1, IL-17, IL-18, Th2, IL-12, IL-23) to induce immunity. Prof. Boissier concluded by saying that the use of therapeutic vaccines for the treatment of RA is a highly potent strategy that offers the advantage of fewer treatments that are administered over longer time periods and has a favorable benefit/risk ratio.
CD4+CD25+ Regulatory T Cells Inhibit Inflammation By Shedding Soluble TNFRII
Hans-Ulrich Scherer, MD, PhD, Charité—University Medicine Berlin, Berlin, Germany, presented new results of research that showed that Treg cells that were adoptively transferred into mice with collagen-induced arthritis slowed disease progression by decreasing systemic mediators of inflammation, independent of an interaction with effector T cells [Morgan ME et al. Arthritis Rheum 2005]. As an explanation of how TNF-blocking therapy might be effective in human arthritis patients, the study showed that murine and human Treg cells inhibit inflammation by secreting soluble TNF (TNFR-II) that blocks TNF-α.
- © 2008 MD Conference Express