Summary
This article discusses best practices for SLE treatment including ways to re-establish immunebalance, early B Cell repopulation, the management of the antiphospholipid antibody syndrome, and new progress in the management of lupus nephritis.
- lupus
Re-Establishing Immune Balance
David Isenberg, MD, PhD, Royal Free/University College Medical School, London, UK, discussed a regimen that he uses to treat SLE patients who have failed all prior treatments. The regimen consists of 2 doses of rituximab (1 g) plus 125–250 mg methylprednisolone 2 weeks apart, followed 24 hours later by 750 mg cyclophosphamide. During this regimen, all prior immunosuppressives are stopped until CD19 counts return to normal; hydroxychloroquine and steroids (10–15 mg) are continued.
To date, 50 patients (mean follow-up after B cell depletion, 39.6 months) have been treated. Of the 45 patients who remained in the study as of the end of 2007, 47% had not flared. Of the 53% who had flares, 5 flared within 6 months, 13 between 6–12 months, and 6 after 12 months of treatment. Nineteen patients were retreated safely (15 had 2 cycles; 4 had 3 cycles). The median time to repopulation (CD19 count >0.03 × 109/L) was 6 months. One patient remains B cell-depleted 90 months after initial treatment.
Prof. Isenberg identified several questions that remain to be answered concerning B cell depletion, including: what determines when the B cells repopulate, why the time to B cell return is so variable, and why the variation in time between the return of B cells and the return of clinical features is so marked.
Early B Cell Repopulation Predicts Relapse After Rituximab in SLE
The ability to identify when B cell repopulation has started may provide an early retreatment opportunity and thus prevent relapse. Edward Vital, MD, ChB, University of Leeds, Leeds, UK, presented results from a study that evaluated whether using rare event flow cytometry (RE-FACS) to count peripheral blood B cells can predict response and relapse after rituximab therapy in patients with SLE.
Patients (n=27) with active SLE that was refractory to standard therapies were treated with 2 infusions of 1 g of rituximab as well as oral and IV steroids. Response was defined as no BILAG A or B; relapse as BILAG A or B after initial response. RE-FACS was performed at baseline and at Weeks 2, 6, 14, and 26 using 6-color flow cytometry, counting 2–500,000 events with exclusion markers that permitted reproducible enumeration of B cells at 0.005% (maximum sensitivity 0.002%). Memory and naïve cells were defined using CD27/38 and pre-plasma cells as CD86+CD24-CD38++CD19+/−CD27++IgM-IgD−.
All patients had at least one BILAG A or B at baseline. Twenty-three patients responded to rituximab at 6 months, and 4 did not respond. Time to relapse was, however, more variable; the majority of patients (n=13) demonstrated sustained responses beyond 12 months, and most (n=11) remain in remission after follow-up, ranging from 18 to 45 months. Of those patients with relapse following rituximab (n=8), most relapsed within 12 months (n=6). These outcomes could be predicted using RE-FACS.
Initial depth of depletion was found to be more variable than suggested by previous reports, and it also predicted initial response (ie, at 6 months). While conventional flow cytometry, using a lower limit of detection of 0.005 × 109/L, indicated that only 5% of patients had detectable B cells, RE-FACS detected B cells in 43% of patients following treatment. Response at 6 months appeared more likely when B cells were undetectable. Eighty-seven percent of patients with undetectable B cells responded at 6 months compared with 57% of patients in whom B cells were detected by RE-FACS.
The pattern of early B cell repopulation predicted relapse in responders. While conventional flow cytometry could detect B cells in only 30% of patients at 6 months, RE-FACS detected B cells in 90% of patients. B cell subsets at 6 months, in responders (ie, no BILAG A or B at 6 months), were therefore compared between those who relapsed in the subsequent 6 months and those with sustained remission of more than 18 months. There was a non-significant trend to higher total B cell numbers in patients who relapsed. There were significantly higher numbers of memory (p=0.027) and pre-plasma cells (p=0.002) in patients who relapsed.
According to Dr. Vital, prediction of relapse using RE-FACS may provide an opportunity to intervene to prevent relapse.
Management of the Antiphospholipid Antibody Syndrome
Warfarin is the most effective treatment for the secondary prevention of recurrent thrombosis in antiphospholipid syndrome (APS) patients. Michael Lockshin, MD, Cornell University, New York, NY, discussed several studies that have added to an evidence-based approach to the use of warfarin in this group of patients.
In a study that assessed CYP2C9 genotypes, VKORC1 haplotypes (designated A and non-A), clinical characteristics, response to therapy, and bleeding events in 297 patients who started warfarin therapy, Schwarz and colleagues found that patients who had the A/A haplotype of VKORC1 had a significantly decreased time to first INR within the therapeutic range (p=0.02) and to first INR >4 (p=0.003) versus those who had the non-A/non-A haplotype. The CYP2C9 genotype was a significant predictor of the time to first INR >4 (p=0.03), but not with respect to the time to first INR within the therapeutic range. Both the CYP2C9 genotype and VKORC1 haplotype had a significant influence on the required warfarin dose after the first 2 weeks of therapy [Schwarz UI et al. N Engl J Med 2008].
Several studies examined the efficacy of high (INR 3.1- 4.5) versus moderate or low doses (INR 2.0–3.0) of warfarin as thromboprophylaxis and found that high-intensity is not superior to moderate/low-intensity warfarin in patients with APS and previous thrombosis and, in fact, may be associated with an increased rate of minor hemorrhagic complications [Crowther MA et al. N Engl J Med 2003; Finazzi G et al. J Thromb Haemost 2005].
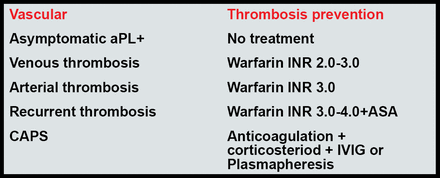
For most patients, Dr. Lockshin recommends that a target INR of 2.5 is as effective as and less dangerous than a target of 3.5. Current recommendations for the treatment of APS are shown in Figure 1.
Current Recommendations for the Treatment of APS.
Erkan, Lockshin. Rheum Dis Clin North Am 2006.
Lim, Crowther, Eikelboom. JAMA 2006.
Progress in the Management of Lupus Nephritis
W. Joseph McCune, MD, University of Michigan, Ann Arbor, MI, reviewed several trials that have improved the ability of clinicians to manage SLE.
Takada and colleagues have shown that certain cytochrome P450 enzyme genotypes may be valuable for predicting the risk of premature ovarian failure in lupus nephritis patients who are treated with cyclophosphamide [Takada K et al. Arthritis Rheum 2004].
Sequential therapy using cyclophosphamide to induce remission, followed by maintenance therapy with a different immunosuppressive, has been shown to reduce toxicity [Contreras G et al. N Engl J Med 2004].
At least 2 studies have shown that race may influence choice of treatment. Dooley and colleagues have reported that in SLE patients who were treated with intravenous cyclophosphamide, renal survival was significantly worse in black compared with white patients. Racial differences were independent of age, duration of lupus, history of hypertension, hypertension control during therapy, and activity or chronicity indices on renal biopsy [Dooley M et al. Kidney Int 1997]. More recently, a large trial that did not show mycophenolate mofetil to be superior to intravenous cyclophosphamide in producing partial or complete remission when patients of all races were considered did show superior results in mycophenolate mofetil-treated individuals of African descent or Hispanic ethnicity.
- © 2008 MD Conference Express