Summary
Given the recent advances in rheumatologic treatment, particularly the advent of TNF therapy, achieving remission or a diminished level of disease activity has become a reasonable treatment objective for patients with rheumatoid arthritis. This article explores the issues of how to define and measure remission and how to manage patients who have achieved remission in daily clinical practice.
- rheumatoid arthritis
Given the recent advances in rheumatologic treatment, particularly the advent of TNF therapy, achieving remission or a diminished level of disease activity has become a reasonable treatment objective for patients with rheumatoid arthritis (RA). This Best Practice Session explored the issues of how to define and measure remission and how to manage patients who have achieved remission in daily clinical practice.
Clinical Remission in Daily Practice
Assessment of remission in RA varies considerably and often is contingent on who is defining it and what scale and cutoff points are being used to measure it. As an example, Daniel Aletaha, MD, PhD, University of Vienna, Vienna, Austria, discussed a study that he conducted, which indicated that more patients would be regarded as being in remission using joint counts (SJC, 35%; TJC, 55%) versus global scores (PGA, 18%; EGA, 9%) and that fewer patients would be regarded as being in remission by physician-derived or laboratory measures than by patient-derived ones [Aletaha D et al. Rheumatology 2006]. According to Prof. Aletaha, regardless of its definition, remission has merit as a treatment goal and should continue to be the measure of the RA disease process and outcome. In addition, he noted that, in his opinion, it is not necessary to define remission as complete absence of disease: “Serving as a treatment target is sufficient.” Beyond the definition or tool that is used for assessment, the level of disease activity should be measured more frequently, clinicians need to be flexible regarding treatment goals in order to improve subsequent treatment decisions, and aiming for sustained remission will improve outcomes, Dr. Aletaha concluded.
Imaging Remission in Daily Practice
Citing the outcomes of several studies, Paul Emery, MD, PhD, University of Leeds, Leeds, UK, discussed the benefits of imaging in the assessment of remission in everyday clinical practice.
Brown and colleagues showed that in asymptomatic, remitted patients who are on disease-modifying antirheumatic drugs (DMARDs) with clinically normal joints, MRI detected synovitis in 96% of patients and bone marrow edema in 46%; ultrasonography detected gray-scale (GS) synovial hypertrophy in 73% of patients, while 43% had increased Power Doppler (PD) signal [Brown AK et al. Arthritis Rheum 2006]. In a follow-up study, only MRI and ultrasonography predicted radiographic progression of RA after 12 months of continued treatment, suggesting that although DMARDs may produce clinical remission, they may not produce true remission [Brown et al. Arthritis & Rheumatism 2008 - In press].
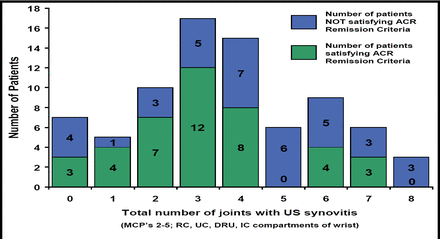
In a study that correlated ultrasonography-detected synovitis and PD signal with DAS28 scores and clinical examination, [Saleem B et al. EULAR 2008 SAT0145] there were no clinical features or clinical remission scores that usefully predicted significant subclinical synovitis (Figure 1). Thus, clinical findings alone cannot predict imaging findings, suggesting the need for sensitive imaging for accurate assessment of RA remission. The difficulty of achieving true remission is demonstrated by the finding that in one current study it was reported that of the 90% of patients with clinical remission, no patients achieved remission when judged by imaging (GS and PD) standards. Clinical remission was reached at around 14 weeks, and although the number of joints with synovitis fell steadily, low levels of synovitis always were present. [Wakefield et al, Arthritis & Rheumatism 2007].
Number of Patients with GS US Synovitis (ACR Remission vs Non ACR Remission).
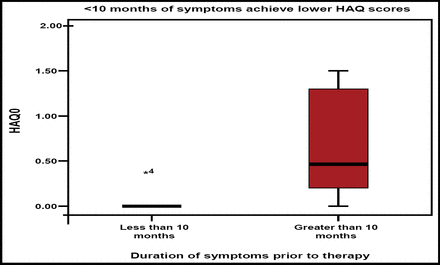
Can biologic-free remission ever be achieved? Prof. Emery stated that early RA treatment with TNF blockers plus methotrexate offers a window of opportunity for achieving biologic-free remission. The major factors that permit successful cessation of anti-TNF blockade are duration of symptoms of <10 months and low levels of imaging synovitis that result in normal HAQ and immunology (Figure 2). He concluded by saying that “imaging is necessary for optimal management of RA and that TNF blockade is more effective than DMARDs in producing remission in more patients, despite the ongoing presence of synovitis.”
Flare vs Sustained Remission in Patients Stopping TNF Blocker Therapy.
How to Manage a Patient in Remission
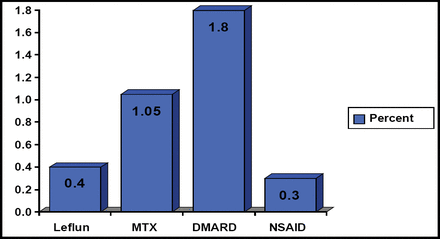
Daniel Furst, MD, University of California, Los Angeles, CA, agreed that the definition of RA remission is variable and changing and that the old definitions were too liberal. He argued that clinicians need to agree on the best definition of remission and test these definitions using registries and ultimately in randomized controlled trials. In the meantime, he suggests choosing a definition and altering therapy as needed based on this definition to produce the best possible outcome. He noted that all of the current treatments have serious side effects. Glucocorticoids are associated with reduced bone density and subsequent osteoporosis, weight gain, and cataracts; DMARDs like methotrexate can interfere with the bone marrow's production of blood cells and increase the risk of liver disease; and anti-TNF therapies can increase the risk for serious infections and liver abnormalities. All of these treatments can increase the risk of death to varying degrees (Figure 3). Dr. Furst recommends the following process for the management of RA treatment: decrease and stop steroid use first, decrease or stop nonbiologic DMARDs next, then reduce the dose and spread the duration of biologic administration. He admits that this management approach remains untested.
Diane van der Woude, MD, Leiden University Medical Center, Leiden, The Netherlands, presented the results of a retrospective study that evaluated DMARD-free remission in RA patients who presented to the Leiden Early Arthritis Clinic who were treated with a delayed (1993–1995) or early treatment strategy using chloroquine, sulfasalazine, or methotrexate (1996–2002). DMARD-free remission was defined as persistent (>1 year) absence of synovitis without concurrent use of DMARDs and subsequent discharge from the outpatient clinic.
Increased Risk of Mortality.
During an average follow-up of 8.2 years, 69 of 454 (15.2%) patients achieved DMARD-free remission (15.2%). Univariate analysis revealed that the following factors were significantly associated with achieving DMARD-free remission: negative family history (HR of 1.8), short duration of complaints before presentation (HR 1.08 per month), nonsmoking (HR 1.8), low CRP at baseline (HR 1.01 per mg/L), absence of IgM rheumatoid factor and anti-CCP antibodies (HR 5.9 and 11.6, respectively), and absence of HLA-shared epitope alleles (HR 2.1). Multivariate analysis revealed that the following factors were significant independent predictors for achieving DMARD-free remission: low CRP at baseline and absence of anti-CCP antibodies.
- © 2008 MD Conference Express