Summary
This article discusses results of 2 48-week, randomized, double-blind, placebo-controlled clinical trials that evaluated the humanized anti-CD22 monoclonal antibody epratuzumab for the treatment of patients with severe BILAG A or moderate BILAG B (in =2 organ systems) systemic lupus erythematosus flares.
- rheumatology clinical trials
- lupus
Michelle Petri, MD, MPH, Johns Hopkins University, Baltimore, MD, reported the results of 2 48-week, randomized, double-blind, placebo-controlled clinical trials that evaluated the humanized anti-CD22 monoclonal antibody epratuzumab for the treatment of patients with severe BILAG A or moderate BILAG B (in ≥2 organ systems) SLE flares.
Patients were randomly assigned to receive placebo (n=37), epratuzumab 360 mg/m2 (n=42), or epratuzumab 720 mg/m2 (n=11) infusions for up to 4 treatment cycles. Corticosteroids were increased by ≥10 mg daily at baseline. Other SLE-specific therapies remained unchanged. Systematic tapering of corticosteroids was started at Week 4 with the aim of achieving a reduction in daily corticosteroid doses to ≤10 mg daily (Study 1) or ≤7.5 mg daily prednisone equivalents (Study 2) by Weeks 20–24. The primary study endpoint was reduction at Week 12 of all BILAG A to B and BILAG B to C, no worsening in other systems, and no addition or increase in immunosuppressives and/or anti-malarials or corticosteroids above baseline or specified taper levels. These studies were prematurely discontinued due to study drug supply interruption, and the data from the 2 trials were combined for analysis. Patients were followed for 6 months after enrollment or cessation of dosing. Patients had varying lengths of study participation and numbers of doses.
Patients were predominately women (94%), who had a mean age of 36.8 years. At baseline, >40% of patients had BILAG A, and 91% had >2 BILAG As or Bs with a mean total BILAG score of 13.2. Over 60% were receiving immunosuppressives; 43% of patients were receiving >25 mg/day corticosteroids.
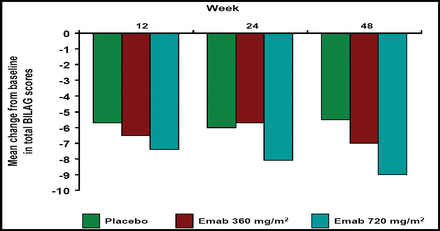
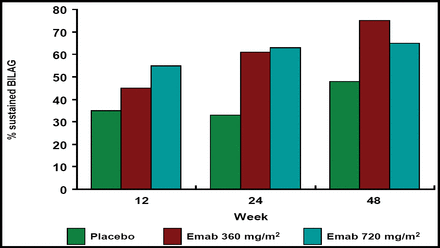
Epratuzumab at both doses resulted in greater reductions in total BILAG scores versus placebo, and the probability of achieving a sustained response prior to Week 12, 24, and 48 was greater with epratuzumab treatment (Figure 1A and B). Patients who received epratuzumab were twice as likely as placebo-treated patients to achieve sustained improvement in BILAG scores (HR 2.18; p=0.021; Table 1). BILAG scores and global disease assessments of disease activity at Week 12 are summarized in Table 1. Cumulative corticosteroid use over 24 weeks was lower in epratuzumab-treated patients versus placebo-treated patients.
Mean Change from Baseline in Total BILAG Scores.
Percent of Sustained BILAG.
Week 12 BILAG Scores and Global Disease Assessments (ITT Population).
The most commonly reported adverse event (AE) overall was upper respiratory infection, which occurred in 35% of patients in the placebo group, 20% of patients in the epratuzumab 360 mg group, and 27% of patients who were treated with epratuzumab 720 mg. The most common AEs (≥10% incidence with epratuzumab) included headache, arthralgia, nausea, pyrexia, abdominal pain, oral candidiasis, peripheral edema, chest pain, cough, and blurred vision. The incidence of SAEs, infusion-related AEs, and infections was similar between placebo and epratuzumab treatment groups.
Treatment with both doses of epratuzumab resulted in clinically meaningful efficacy, as evidenced by improvements in BILAG, physician and patient global assessment, and clinically meaningful corticosteroid sparing in patients with moderate and severe SLE flares. “These initial clinical results for epratuzumab are very encouraging,” said Dr. Petri. “Developing new compounds for SLE patients is critical because currently available treatments, such as immunosuppressants and corticosteroids, often have serious and debilitating side effects. We look forward to seeing results from other clinical trials involving epratuzumab.”
- © 2008 MD Conference Express