Summary
Although studies have shown current anti-TNF treatments to be superior to short-term methotrexate (ie, before 24 weeks), none has shown superiority at Week 24. The AMBITION study [NCT00109408] showed that after 24 weeks, tocilizumab (an anti-IL-6 receptor antibody that inhibits IL-6 signaling) monotherapy was clinically superior to methotrexate (MTX) monotherapy in patients with rheumatoid arthritis who have not failed previous MTX or biologic treatment.
- rheumatology clinical trials
- rheumatoid arthritis
Although studies have shown current anti-TNF treatments to be superior to short-term methotrexate (ie, before 24 weeks), none has shown superiority at Week 24. Graeme Jones, MD, PhD, University of Tasmania, Hobart, Australia, lead investigator of the AMBITION study (NCT00109408), presented data that showed that after 24 weeks, tocilizumab (an anti-IL-6 receptor antibody that inhibits IL-6 signaling) monotherapy was clinically superior to methotrexate (MTX) monotherapy in patients with rheumatoid arthritis (RA) who have not failed previous MTX or biologic treatment.
AMBITION was a randomized, double-blind, placebo-controlled, phase 3 study in patients with active moderate to severe RA of at least 3 months duration who were MTX-naïve or had not received MTX for at least 6 months before randomization and had not previously failed MTX or biologic treatment. Additional inclusion criteria included: swollen joint count ≥6 (of 66) and tender joint count ≥8 (of 68) at screening/baseline and CRP ≥1.0 mg/dL or ESR ≥28 mm/hr. Patients were randomly assigned to receive either tocilizumab 8 mg/kg every 4 weeks plus placebo capsules weekly or placebo infusions every 4 weeks plus methotrexate (7.5 mg/week titrated to 20 mg/week within 8 weeks).
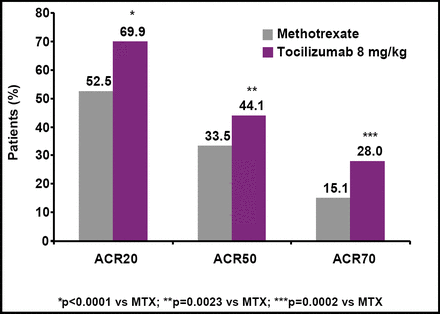
After 24 weeks of treatment, 70% of tocilizumab patients achieved a 20% improvement in their symptoms versus 53% of MTX patients (p<0.0001; Figure 1). Significant differences in favor of tocilizumab also were seen on ACR50 and ACR70 responses (Table 1). A higher proportion of patients who received tocilizumab achieved a good/moderate EULAR response as early as Week 2 (64% vs 19% MTX).
Mean change in CRP levels from baseline to Week 24 was −2.6 mg/dL for tocilizumab versus −1.9 mg/dL for MTX. The incidence of adverse events (AEs) was similar (80% tocilizumab; 78% MTX). AEs that led to withdrawal were more common in the MTX arm (5.3% vs 3.8% for tocilizumab). Serious AEs were higher with tocilizumab (4% vs 3% for MTX), as were serious infections (1.4% vs 0.7% for MTX), but these differences were not statistically significant. Shifts in ALT from normal at baseline to >3x ULN occurred more frequently in the MTX arm (4%) compared with tocilizumab (2%). Shifts in total cholesterol from <200 to >240 mg/dL occurred more frequently with tocilizumab (13% vs <1% for MTX).
ACR 20, 50, and 70 Response Rates at Week 24.
Results at Week 24 (ITT population).
“We are very encouraged by the results of the AMBITION study, which shows for the first time that treatment with a single biologic agent is superior to methotrexate at 6 months of therapy,” said Prof. Jones. “Overall, these compelling results further establish the efficacy and safety of tocilizumab in treating the chronic signs and symptoms of rheumatoid arthritis that dramatically affect the lives of patients.”
- © 2008 MD Conference Express