Summary
This article discusses the initial 52-week data from the COMET trial which showed that etanercept in combination with methotrexate (MTX) was superior to MTX alone in providing clinical remission, radiographic non-progression, and normalized function.
- rheumatoid arthritis clinical trials
The COMET trial, the first biologic rheumatoid arthritis (RA) clinical trial to use remission as a primary endpoint, is a 2-year double-blind, randomized trial that included active RA patients (DAS28 ≥3.2) with a disease duration ≥3 months and ≤2 years who were methotrexate-naïve. Patients received either etanercept plus methotrexate (MTX) or MTX monotherapy. MTX was titrated beginning at Week 4 to a maximum of 20 mg/week at Week 8. The primary study efficacy endpoints were the proportion of patients achieving DAS28 remission (<2.6) and change from baseline in modified Total Sharp Score (mTSS) at Week 52. Other endpoints included low disease activity (DAS28 ≤3.2), radiographic non-progression (mTSS ≤0.5), and percentage of patients achieving a normal HAQ score (≤0.5).
Paul Emery, MD, PhD, University of Leeds, Leeds, UK, presented the initial 52-week data from the COMET trial which showed that etanercept in combination with MTX was superior to MTX alone in providing clinical remission, radiographic non-progression, and normalized function.
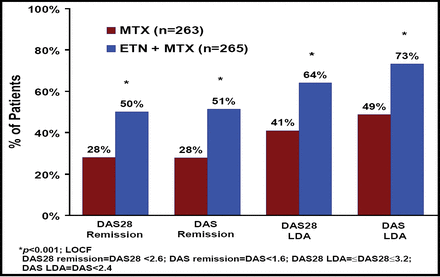
Data for a total of 528 patients were evaluable for clinical efficacy (etanercept+MTX, n=265; MTX alone, n=263) and for 476 patients for radiographic efficacy (etanercept+MTX, n=246; MTX alone, n=230). At Week 52, significantly (p<0.001) more patients in the etanercept+MTX group (50%; 132/265) achieved DAS28 remission versus in the MTX alone group (28%, 73/263; Figure 1). A significantly (p<0.001) greater proportion of patients in the combination therapy group (64%) also achieved DAS28 ≤3.2 versus the MTX alone group (41%; Figure 1).
DAS28 and DAS Remission and Low Disease Activity (LDA).
Combination therapy also resulted in significantly (p<0.001) lower radiographic progression than MTX monotherapy (mean mTSS changes from baseline: 0.27 for etanercept+MTX and 2.44 for MTX alone). Radiographic non-progression was achieved by 80% (196/246) of patients receiving combination therapy and 59% (135/230) receiving MTX alone (p<0.001). The proportion of patients achieving HAQ ≤0.5 was significantly (p<0.001) greater in the combination group (55%, 140/256) versus the MTX group (39%, 93/241).
Serious adverse events were reported by 33 patients (12%) in the combination group and 34 (13%) in the MTX group. There were no differences in the rates of serious infections or malignancies and no cases of tuberculosis or demyelinating disease.
“Until recently, we did not know whether remission was a realistic”, said Prof. Emery. “We now have results which show that not only is clinical remission achievable in a significant number of patients, but radiographic and functional remission are also achievable. These exciting results lead to the next therapeutic step in aiming for multiple measures of remission as our treatment goal, no longer just one”.
- © 2008 MD Conference Express