Summary
Adjuvant 5-FU-based chemotherapy regimens have improved survival for patients with colorectal cancer metastases to the liver compared with surgery alone. The findings of an international, randomized phase 3 trial [NCT00143403] indicate that the addition of irinotecan to 5-FU/leucovorin did not lead to further improvement in this setting.
- Adjuvant/Neoadjuvant Therapy
- Gastrointestinal Cancers
Adjuvant 5-FU-based chemotherapy regimens have improved survival for patients with colorectal cancer metastases to the liver compared with surgery alone. The findings of an international, randomized phase 3 trial (NCT00143403) indicate that the addition of irinotecan to 5-FU/leucovorin did not lead to further improvement in this setting.
“No difference was observed in disease-free survival (DFS) between the 2 arms, adjusted for important prognostic factors or not,” said Marc Ychou, MD, Centre Regional de Lutte contre le Cancer Val d'Aurelle, Montpellier, France, who presented the findings of the study.
Patients were randomly assigned to either 5-FU/leucovorin alone or 5-FU/leucovorin plus irinotecan (FOLFIRI) within 3–8 weeks after surgical resection of liver metastases. There were 153 patients in each arm. Stratification factors included number of liver metastases, prior adjuvant chemotherapy, and time between resection of primary tumor and diagnosis of liver metastasis. The primary endpoint was DFS, and secondary endpoints included safety and OS. The median follow-up was 42 months.
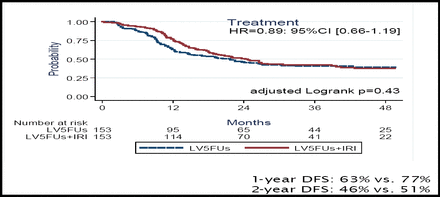
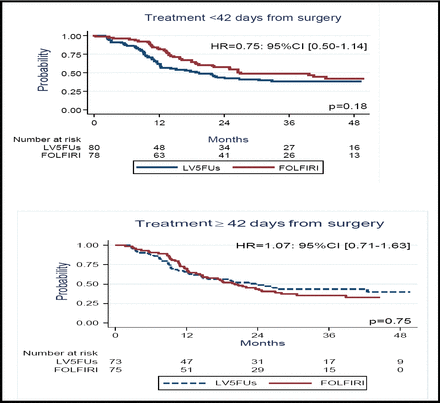
Dr. Ychou reported that the median DFS did not differ significantly between the 2 arms (21.6 months for 5-FU/leucovorin vs 24.7 months for FOLFIRI; p=0.43). The 1-year and 2-year DFS also did not differ significantly (Figure 1A). At 1 year, the DFS rate was 77% for the FOLFIRI arm and 63% for 5-FU/leucovorin alone; at 2 years, the corresponding rates were 51% and 46%. Exploratory analysis indicated that there was a trend toward improved DFS when treatment with FOLFIRI was started within 42 days after surgery (Figure 1B). The 3-year OS was similar for the 2 arms (73% for FOLFIRI vs 72% for 5-FU/leucovroin).
The 1-Year And 2-Year DFS Did Not Differ Significantly Between The 2 Arms.
Exploratory Analysis Indicated That There Was A Trend Toward Improved DFS When Treatment With FOLFIRI Was Started Within 42 Days After Surgery.
With regard to prognostic factors, Dr. Ychou noted that multivariate analysis indicated that a time of 1 year or less between resection of the primary tumor and the diagnosis of liver metastasis was associated with a hazard ratio (HR) of 1.69 (p=0.004). The HR was 1.35 for more than one liver metastasis (p=0.052) and 1.39 for prior adjuvant therapy (p=0.052).
The safety profiles of both treatments were as expected, said Dr. Ychou. The overall grade 3–4 toxicity was significantly higher for the FOLFIRI arm (p=0.01). Toxicity led to a significantly greater number of dose reductions in the FOLFIRI arm (49% vs 18%; p<0.001), resulting in a lower median relative dose intensity of both 5-FU (89% vs 95%) and leucovorin (84% vs 91%) in that arm.
“No [bitherapy] has yet been proven superior to 5-FU alone,” said Dr. Ychou, in closing. “We urgently need large trials in this setting that integrate tailored chemotherapy and targeted therapies.”
- © 2008 MD Conference Express