Summary
The results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial [NCT00004931] demonstrated that oxaliplatin prolongs both progression-free and overall survival for patients with stage II or stage III colorectal cancer. The trial was designed to evaluate the efficacy of oxaliplatin when it was added to bolus 5-FU/leucovorin as adjuvant treatment.
- Gastrointestinal Cancers
The results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial (NCT00004931) demonstrated that oxaliplatin prolongs both progression-free and overall survival (OS) for patients with stage II or stage III colorectal cancer. The trial was designed to evaluate the efficacy of oxaliplatin when it was added to bolus 5-FU/leucovorin as adjuvant treatment.
Norman Wolmark, MD, Chairman of the NSABP, Pittsburgh, PA, who reported the findings, noted that the study protocol called for formal analysis of OS data 5 years after the last patient was entered (November 2002). The number of deaths that actually occurred (560) was lower than the expected number (700), which reduced the protocol-specified power to detect a 0.214 reduction in the annual death rate.
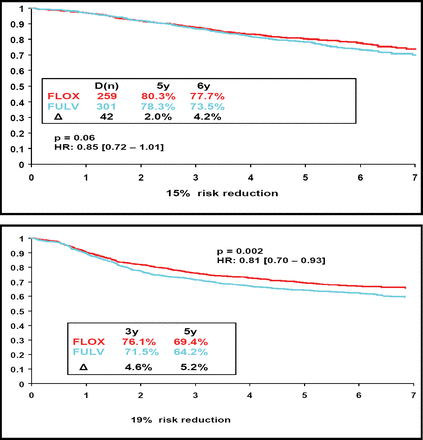
Dr. Wolmark reported that oxaliplatin was associated with a trend toward better OS at both 5 years and 6 years, a difference of borderline significance (Figure 1A). In addition, the updated results on disease-free survival (DFS) showed that the effect of oxaliplatin was durable. At a mean follow-up of 67 months, there was a 19% reduction in the risk of disease progression (Figure 1B).
The Combination of Oxaliplatin Plus Bolus 5-FU/Leucovorin (FLOX) Led to Better OS (1A) and DFS (1B) Than 5-FU/Leucovorin Alone (FULV).
Dr. Wolmark noted that the findings confirm the results of the Multicenter International Study of Oxaliplatin/Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) trial, in which infusional combination fluorouracil and leucovorin was evaluated with and without oxaliplatin (Andre et al. N Engl J Med 2004). The 5-year survival for the oxaliplatin-containing regimen in the MOSAIC trial was 81.3%, which represented an absolute difference of 2.2% (compared with 80.3% and 2.0%, respectively, in the C-07 trial). The hazard ratio for this difference was 0.85 in both studies. “The findings indicate that the benefit of oxaliplatin in terms of both overall and disease-free survival is independent of the schedule of administration [of 5-FU],” said Dr. Wolmark.
In discussing the appropriate length of follow-up for colorectal cancer trials, Dr. Wolmark pointed out that the median survival after recurrence in NSABP trials has improved significantly over time. For example, the median survival after the C-04 trial (which began enrolling patients in 1989) was 12.7 months, compared with 19.6 months for the C-07 trial, which began enrolling patients in 2000 (p<0.0001). This finding, along with the significant increase in the absolute advantage of oxaliplatin in the C-07 trial, indicates that future trials of adjuvant therapy should utilize longer follow-up (beyond five years) in order to reliably detect differences in OS, he said.
- © 2008 MD Conference Express