Summary
In patients with grossly serosa-positive advanced gastric cancer, postoperative adjuvant chemotherapy with intraperitoneal cisplatin and early mitomycin C (M) plus long-term doxifluridine plus cisplatin (iceMFP) improved recurrence-free and overall survival. The comparator regimen in the phase 3 trial [AMC 0101] was M plus short-term doxifluridine.
- Gastrointestinal Cancers Clinical Trials
In patients with grossly serosa-positive advanced gastric cancer (AGC), postoperative adjuvant chemotherapy with intraperitoneal cisplatin and early mitomycin C (M) plus long-term doxifluridine plus cisplatin (iceMFP) improved recurrence-free and overall survival (OS). The comparator regimen in the phase 3 trial (AMC 0101) was M plus short-term doxifluridine (Mf), according to Yoon-Koo Kang, MD, Asan Medical Center, Seoul, Korea.
Dr. Kang noted that small but significant benefit for adjuvant chemotherapy in AGC has been demonstrated in meta-analyses and that a meta-analysis of M-based adjuvant chemotherapy studies from the 1960s-1980s in Japan (Nakajima et al. Gan To Kagakis Ryoho 1994) also demonstrated efficacy. The clinical intent of the active therapy in trial AMC 0101 was to improve adjuvant chemotherapy with M and short-term oral fluoropyrimidine by adding cisplatin, prolonging the administration of low-dose fluoropyrimidine, starting chemotherapy early, and using an intraperitoneal administration route.
The trial tested whether these strategies would improve recurrence-free survival (RFS) and, secondarily, OS. It included curatively resected AGC performance status II-IV patients without metastases who were randomized at surgery to either of 2 arms: Mf or iceMFP. The Mf group received 20 mg/m2 of M injected 3–6 weeks after surgery and 4 weeks later, 460–600 mg/m2/day of doxifluridine administered orally for 3 months. For the iceMFP group, 100 mg of cisplatin in 1 L of saline was administered intraperitoneally for 2 hours during surgery, and 15 mg/m2 of M was injected 1 day after surgery. Doxifluridine was started 4 weeks after surgery and extended for a total of 12 months. Six shots of monthly 60 mg/m2 cisplatin were added.
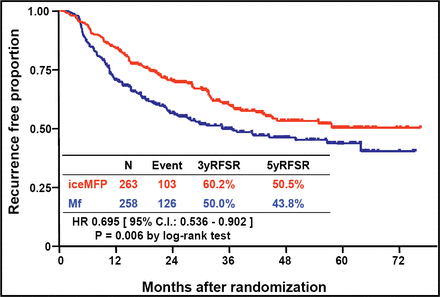
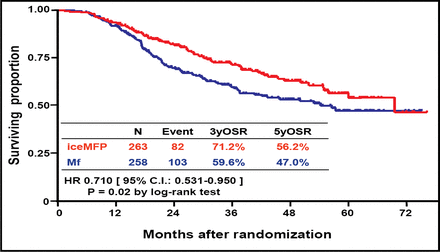
Among 521 patients who were analyzed (mean age ∼54.5 years, 67% male), after a median follow-up of 3.5 years, RFS favored the iceMFP arm significantly (HR 0.695; p=0.006; Figure 1). Five-year RFS was 50.5% in the iceMFP arm and 43.8% in the MF arm. OS also favored the iceMFP arm (HR 0.710; p=0.02; Figure 2), whereby the 5-year OS was 56.2% for iceMFP and 47.0% for Mf. Surgery-related complications were generally similar between groups.
RFS at 3.5 Years.
RFS at 5 Years.
Dr. Kang also noted that recurrences were significantly less frequent in the iceMFP arm (94 vs 1184; p=0.02) and that the reduction of recurrences was observed not only in the peritoneum but also in other local or distant sites to a similar extent. Grade 3–4 neutropenia was more frequent in the iceMFP arm (34.2% vs 11.6%).
Dr. Kang concluded, “Postoperative iceMFP chemotherapy was safe and significantly improved RFS and OS in patients with grossly serosa-positive AGC compared with Mf chemotherapy.” He added, “Considering that adding cisplatin and prolonging oral doxifluridine provided no benefit in another trial, the AMC 0201 trial (Abstract #4531. ASCO 2008), early start of chemotherapy or intraperitoneal cisplatin seemed to be responsible for the improved efficacy in AMC 0101.”
The editors would like to thank the many members of the ASCO 2008 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2008 MD Conference Express