Summary
The addition of a vascular endothelial growth factor antibody and an epidermal growth factor receptor inhibitor to chemotherapy had no benefit in advanced colorectal cancer. In fact, the combination had a significantly negative effect on progression-free survival. This article discusses the results of CAIRO2 [NCT00208546], a trial that was designed to investigate the effect of adding cetuximab to chemotherapy plus bevacizumab.
- Oncology Genomics
- Gastrointestinal Cancers
The addition of a vascular endothelial growth factor (VEGF) antibody and an epidermal growth factor receptor (EGFR) inhibitor to chemotherapy had no benefit in advanced colorectal cancer. In fact, the combination had a significantly negative effect on progression-free survival (PFS).
Cornelis J. Punt, MD, PhD, University Medical Center, St. Radboud, Nijmegen, The Netherlands, representing the Dutch Colorectal Cancer Group (DCCG), presented the results of CAIRO2 (NCT00208546), a trial that was designed to investigate the effect of adding cetuximab (CTX) to chemotherapy plus bevacizumab. The trial included 736 eligible patients with previously untreated disease who were randomly assigned to receive capecitabine, oxaliplatin, and bevacizumab or the same schedule plus cetuximab. The primary endpoint was PFS. Secondary endpoints included overall survival (OS), response rate, disease control, and toxicity. The median duration of follow-up was 18.7 months.
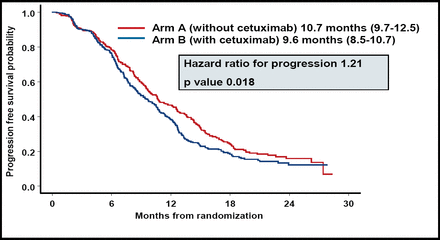
Dr. Punt reported that CTX significantly decreased the median PFS (Figure 1). OS, response rate, and disease control were comparable for the 2 groups (Table 1).
Comparison of Efficacy Results Patients Treated With Capecitabine and Oxaliplatin Plus Bevacizumab, With and Without CTX.
The Addition of CTX to Capecitabine and Oxaliplatin Plus Bevacizumab Significantly Decreased PFS Compared With Patients Treated With the Same Chemotherapy Regimen Without CTX.
The overall rate of grade 3–4 toxicity was significantly higher for the CTX-treated patients (82% vs 72%; p=0.0013), but Dr. Punt noted that when CTX-related skin toxicity was excluded, there was no difference between the 2 groups (75% and 72%, respectively; p=0.37). There also were no significant differences between the 2 treatment groups with regard to the frequency of any other adverse events, except for diarrhea, which occurred significantly more often among patients who were treated with CTX (26% vs 19%; p=0.026). All-cause mortality within 30 days or less of the last treatment was similar for the 2 groups (5%), as well as the 60-day all-cause mortality (2.4% vs 1.9%).
Dr. Punt noted that the results of the study also were evaluated with regard to KRAS status. He reported that among the 196 patients who had the KRAS mutation, the PFS was significantly decreased for patients who were treated with CTX (8.6 months vs 12.5 months; p=0.043). KRAS status had no effect on OS. Patients with wild-type KRAS status did not benefit from CTX, as has been shown in other studies with chemotherapy and CTX or with CTX alone.
Cathy Eng, MD, MD Anderson Cancer Center, Houston, TX, who discussed the study, pointed out that the findings were comparable with the results of the Panitumumab Advanced Colorectal Cancer Evaluation (PACCE) trial.1 [Hecht et al. 9th World Conference on Gastrointestinal Cancers, Barcelona, 2007]. In that study, the PFS that was associated with FOLFOX plus bevacizumab was 11.1 months compared with 9.6 months for FOLFOX plus bevacizumab and panitumumab (p=0.004; HR=1.44). Dr. Eng noted, “[The results of this study and others] indicate that our understanding of both VEGF and EGFR pathways is not fully understood.”
- © 2008 MD Conference Express