Summary
In 2008, the American College of Cardiology and the American Heart Association updated major guidelines that addressed the diagnosis, risk stratification, and treatment of conditions that span the range of acute coronary syndromes, from unstable angina and non-ST-elevation myocardial infarction to ST-elevation myocardial infarction.
- cardiology guidelines
- interventional techniques & devices
- thrombotic disorders
- myocardial infarction
Within the past year, the American College of Cardiology (ACC) and the American Heart Association (AHA) have updated major guidelines that address the diagnosis, risk stratification, and treatment of conditions that span the range of acute coronary syndromes (ACS), from unstable angina (UA) and non-ST-elevation myocardial infarction (NSTEMI) to ST-elevation myocardial infarction (STEMI).
On August 6, 2007, the ACC and AHA published updated guidelines on the management of patients with UA/NSTEMI [Anderson et al. Circulation 2007]. On December 10, 2007, two focused guideline updates were released; the first addressed the management of patients with STEMI [Antman et al. Circulation 2008], and the second addressed treatment decisions related to percutaneous coronary intervention (PCI) [King et al. J Am Coll Cardiol 2008].
In this session at the ACC annual meeting, four leading experts in ACS and authors involved in developing the new guidelines provided insights on key updates.
Antithrombotic Therapy for NSTEMI
Jeffrey L. Anderson, MD, University of Utah School of Medicine, Salt Lake City, UT, focused on the “delicate balance between efficacy and bleeding risk” associated with antithrombotic strategies for patients with NSTEMI.
“In every major epidemiologic study, bleeding—particularly major bleeding—has been shown to have a major impact on cardiovascular outcomes,” Dr. Anderson said. “At least 15% of the excess major bleeding can be attributed to administering antithrombotic agents at the incorrect doses,” he said.
According to Dr. Anderson, antithrombotic therapy dosing errors—and the bleeding events that they may cause—are far too prevalent. In the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC and AHA Guidelines (CRUSADE) registry, 42% of patients with NSTEMI who were given antithrombotic agents received at least one initial dose outside the recommended range [Alexander et al. JAMA 2005].
Dosing errors are particularly common when physicians are using unfractionated heparin (UFH), low-molecular weight heparin (LMWH), and glycoprotein IIb/IIIa inhibitors. Often, these agents require dose adjustment based on body weight and renal function. Therefore, patients with lower body weight, such as women and those with renal insufficiency, are particularly vulnerable to excess dosing.
Results from the CRUSADE registry were practice-changing, Dr. Anderson said. The special dosing requirements for women and patients with renal insufficiency are highlighted in the new guidelines (Figure 1). Similar cautions are listed for patients with baseline chronic kidney disease.
Updated ACC/AHA Recommendation on the Use of Antithrombotic Therapy in Women with NSTEMI. Anderson et al. Circulation 2007; 116:803–877. Copyright © 2008 American College of Cardiology Foundation and the American Heart Association, Inc.
“There is an important piece of the puzzle regarding the relationship between bleeding and risk that has not yet been solved,” said Elliott M. Antman, MD, Brigham and Women's Hospital, Boston, MA. “That is: What are the causes of death over the long term in people who had an acute bleed? This question has important implications for therapeutic decision-making,” Dr. Antman continued.
If the link between an acute bleed and a long-term adverse outcome can be confirmed, using agents that are less likely to cause bleeding is important, Dr. Antman explained. On the other hand, “if the bleed is simply identified in someone who is high-risk and is destined to have an adverse outcome, I believe that the therapeutic decision-making may be different,” Dr. Antman said.
“Certainly, all of us have to agree that bleeding is a bad complication, and it has some serious implications—whether it explains all or just part of the excess risk,” Dr. Anderson said. The updated UA/NSTEMI guidelines were developed to help physicians minimize the risk for bleeding in this patient population, Dr. Anderson concluded.
Timing of Reperfusion Therapy for STEMI
Eric R. Bates, MD, University of Michigan, Ann Arbor, MI, described the updated recommendations related to reperfusion therapy—including pharmacologic reperfusion and primary PCI—for patients who experience an episode of STEMI.
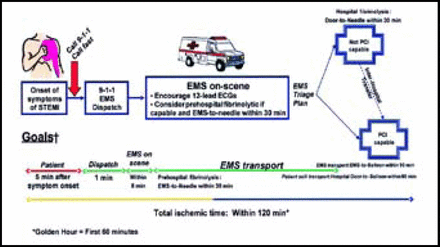
“One of the most controversial points in the guidelines for STEMI has been the timing of reperfusion therapy,” Dr. Bates said. Indeed, the most dramatic update of the STEMI guidelines focuses on the first 60–120 minutes following symptom onset.
“There is a ‘golden hour’ for reperfusion therapy for myocardial infarction (MI), just like there is a golden hour for shock,” Dr. Bates said. “If one can get treatment initiated within the golden two hours—which is probably more feasible than one hour—that is a wonderful treatment strategy with excellent outcomes,” he said.
The updated STEMI guidelines outline the optimal timing of treatment for patients, beginning at the onset of symptoms (Figure 2). The goals include contact between the patient and emergency medical services (EMS) within five minutes of symptom onset, dispatch of EMS within one minute of the EMS call, and arrival of EMS to the patient's location within eight minutes.
Recommended Timing of Patient Assessment and Treatment Initiation in STEMI.
Antman et al. Circulation 2008;117:296–329. Copyright © 2008 American College of Cardiology Foundation and the American Heart Association, Inc.
“The biggest problem we have is getting patients to access the health care system [quickly],” Dr. Bates said. Education regarding the symptoms of MI has decreased the degree to which patients rationalize their symptoms—patients may tell themselves that they are not having a heart attack, despite characteristic signs and symptoms—and thus fail to call EMS. However, despite these gains, many patients still do not call for help in a timely manner. In addition, more than half of patients with STEMI symptoms are being driven to the hospital by a friend or loved one. This postpones vital treatment that EMS, had they been called for help, would be able to initiate prior to arriving at the hospital. Nitroglycerin and aspirin, which are recommended at first medical contact, are now routinely administered by EMS en route to the hospital.
Most of the deaths related to STEMI occur within the first hour of symptom onset, Dr. Bates explained, and “there is still a time delay in getting contact with a defibrillator, which is the most important intervention that we have to offer.”
Once EMS is on the scene, they are encouraged to use 12-lead electrocardiogram (ECG) and consider pre-hospital fibrinolytic therapy. Ideally, patients should have fibrinolytic therapy initiated within 30 minutes of EMS contact and should be undergoing primary PCI within 90 minutes. Patients who are taken to facilities that do not perform PCI should be transferred to PCI-capable hospitals.
“There's no question that reperfusion should be given as soon as possible. The best reperfusion strategy might be the one that can be initiated the quickest,” Dr. Bates concluded.
Interventional Strategies After Admission for STEMI and NSTEMI
Judith S. Hochman, MD, MA, New York University, New York, NY, emphasized the importance of risk stratification in the treatment of patients with NSTEMI or STEMI.
The updated UA/NSTEMI guidelines describe risk stratification as an “integral prerequisite to decision-making” (Level of Evidence: Class I). Moreover, risk stratification is an ongoing process that includes intensive initial patient assessment coupled with ongoing assessment throughout clinical treatment. Targeted ECG and biomarker data are used at each stage of the process.
In the new guidelines, clinicians are asked to approach risk stratification with two questions in mind. First, what is the probability of having obstructive coronary artery disease (CAD) based on the patient's history of ischemia and presenting symptoms? Second, given the presence of obstructive CAD and the diagnosis of ACS, what is the risk of an adverse clinical outcome?
“Risk stratification is very important for selecting a management strategy, and it needs to be done up front. Based on that, you proceed with an initial invasive strategy or an initial conservative strategy,” Dr. Hochman said.
The routine use of risk scores for risk stratification also is a new recommendation in the updated UA/NSTEMI guidelines. According to the guidelines, “risk scores should be a routine part of assessment throughout the hospital course and periodically after discharge” (Level of Evidence: Class IIa B). The Thrombolysis in Myocardial Infarction (TIMI) risk score (Table 1) and the Global Registry of Acute Coronary Events (GRACE) risk score and nomogram are valid, appropriate tools for the assessment of patients with UA/NSTEMI.
Calculation and Interpretation of TIMI Risk Score for UA/STEMI.
For both STEMI and NSTEMI, “there is a spectrum from very stable, low-risk patients to unstable, high-risk patients, based on symptoms, anatomy, and ischemia,” Dr. Hochman said. For these patients, initial risk stratification is an essential component of clinical management. “That's what's going to guide you, and that's how the guidelines are developed based on the evidence,” she said.
Complete guideline information is available at http://circ.ahajournals.org/cgi/reprint/CIRCULATIONAHA.107.185752
The editors would like to thank the many members of the ACC 2008 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2008 MD Conference Express