Summary
This article reviews multiple studies that evaluated other pharmacologic agents, as well as discusses three anticoagulation management strategies that can be sued during atrial fibrillation (AF) ablation and methods for assessing and identifying stroke risk in AF patients.
- cerebrovascular disease
- arrhythmias
- thrombotic disorders
Stating that “There is need for alternatives to warfarin,” Andrew E. Epstein, MD, Mt. Sinai School of Medicine, New York, NY, reviewed two studies that evaluated other pharmacologic agents. The Stroke Prevention in Atrial Fibrillation (SPAF) Study compared 325 mg/day aspirin or warfarin with placebo in atrial fibrillation (AF) patients. Results showed that aspirin and warfarin reduced primary events of death due to ischemic stroke and systemic embolism by 32% (p=0.02) and 58% (p=0.01), respectively, versus placebo. However, bleeding risk between aspirin and warfarin were the same [SPAF Investigators. Circ 1991].
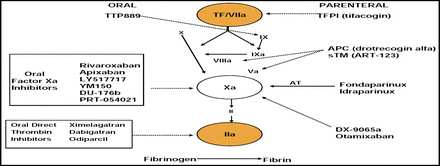
Dr. Epstein also discussed experimental compounds, including the oral direct thrombin inhibitors ximelagatran (Exanta) and dabigatran (PETRO) [SPORTIF III Investigators. Lancet 2003], as well as the factor Xa inhibitors (rivaroxaban and apixaban; Figure 1).
New Anticoagulants.
“In the future, we need to find a drug that beats warfarin's ability to decrease stroke rate to about 1% per year,” said Dr. Epstein, adding that the ideal agent would be available in an oral, fixed-dose form and have rapid onset and offset of action, predictable pharmacokinetics, a low propensity for food and drug interactions, a wide therapeutic window, and no need for monitoring.
Oussama M. Wazni, MD, Cleveland Clinic, Cleveland, OH, discussed 3 anticoagulation management strategies that were used during AF ablation in a trial of patients (n=355) who were undergoing pulmonary vein antrum isolation for persistent AF. Patients in one group discontinued warfarin 3 days prior to ablation, and enoxaparin 1 mg/kg-1 BID SQ was initiated and continued until a therapeutic INR was achieved postprocedure with warfarin. Transesophageal echocardiography (TEE) was performed in one group just prior to the ablation to rule out left atrial thrombus. In the second group, the same algorithm was followed, except the dose of enoxaparin was 0.5 mg/kg-1. In Group 3, the procedure was performed while patients were therapeutically anticoagulated with warfarin to maintain the INR between 2 and 3.5. No enoxaparin was administered
With careful attention before, during, and after ablation, the use of warfarin without enoxaparin was shown to be safe and efficacious. According to Dr. Wazni, this strategy avoids the necessity to administer low-molecular-weight heparin, which lessens patient inconvenience, expense, and the incidence of bleeding.
Dr. Jonathan L. Halperin, Mt. Sinai School of Medicine, New York, NY, stressed the need to identify the level of stroke risk in AF patients before deciding on an approach to anticoagulation therapy, because there is great variability in stroke rate. AF patients at the highest risk are those with mitral stenosis, prosthetic heart valve, left ventricular dysfunction, systolic BP >160 mm Hg, and a history of stroke or TIA. Female gender is an independent risk factor for thromboembolism (and bleeding) and should influence anticoagulant therapy decisions in AF patients.
Current AF guidelines [Fuster. JACC 2006] endorse the use of the CHADS2 risk index (1 point each for CHF, Hypertension, Age > 75 years, Diabetes, and 2 points for Stroke or TIA) to identify patients who are at increased risk for stroke and who should be considered for oral anticoagulation. However, it is not prudent to treat all AF patients with anticoagulants. Low-risk patients (CHADS2 index 0 or 1) can be treated with aspirin, while those with CHADS2 index of 2 or greater might be candidates for warfarin.
Dr. Halperin mentioned the need for better tools to stratify bleeding risk, more precise noninvasive imaging to assess thromboembolism risk, more accurate biomarkers of inflammation and thrombophilia to predict clinical events and guide therapy, and targeted preventive therapy for patients at risk of developing AF as the most important challenges that lay ahead. The goal is “to bring effective therapy to many more patients and prevent thousands of strokes.”
Many patients who undergo coronary artery bypass grafting (CABG) eventually develop AF and are at risk for stroke. Over 90% of thrombi are found in the left atrial appendage (LAA) [Blackshear JL & Odell JA. Ann Thorac Surg 1996]. Surgical occlusion of the LAA is an attractive method for potentially reducing stroke risk and can be done with little incremental time, cost, and risk.
Shephal Doshi, MD, Pacific Heart Institute, Santa Monica, CA, discussed the WATCHMAN, an implantable device that consists of a coated, self-expanding nitinol cage, which is permanently placed at the opening of the LAA, to prevent blood clots from the LAA from entering the bloodstream and potentially causing a stroke.
Based on a successful 5-year event-free pilot study [Sick PB et al. J Am Col Cardiol 2007], the PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Atrial Fibrillation) study is currently comparing the WATCHMAN device with long-term warfarin therapy. The primary endpoints are the rates of all stroke, systemic emboli, and cardiovascular death in high-risk patients who are eligible for warfarin therapy with non-valvular AF. Thus far, 757 patients have been enrolled. First analysis of the data is expected in the summer of 2008.
- © 2008 MD Conference Express