Summary
Final results of the Hypertension in the Very Elderly Trial [HYVET] demonstrated that treatment of hypertension based on indapamide sustained release tended to reduce strokes and all-cause mortality in the elderly.
- hypertensive disease clinical trials
Final results of the Hypertension in the Very Elderly Trial (HYVET) demonstrated that treatment of hypertension based on indapamide sustained release (SR) tended to reduce strokes and all-cause mortality in the elderly. The results were presented at the 2008 American College of Cardiology meeting in Chicago by Nigel S. Beckett, MD, trial coordinator from the Care of the Elderly Group Imperial College, London, UK.
The HYVET trial was a double-blind, placebo-controlled, multicenter, international study in which 3845 persistently hypertensive (mean systolic blood pressure 160–199 mm Hg) men and women aged 80 years and older were randomly assigned to treatment with indapamide SR 1.5 mg tablets once daily (n=1933) or matching placebo (n=1912). The angiotensin-converting enzyme inhibitor perindopril (2 or 4 mg) or matching placebo was added as needed to reach the target blood pressure of 150/80 mm Hg. The primary study endpoint was fatal or nonfatal stroke. Secondary endpoints included death from any cause, death from cardiovascular causes, death from cardiac causes, or death from stroke.
Subjects ranged in age from 80–105 years (73% aged 80–84 years; 22.4% aged 85–89 years; and 4.6% aged ≥90 years). At baseline, mean sitting blood pressure was 173.0/90.8 mm Hg. A total of 452 subjects (about 11.8%) had a previous history of cardiovascular disease; 6.9% of subjects were diabetic. Approximately one-third of patients were naïve to anti-hypertensive medications.
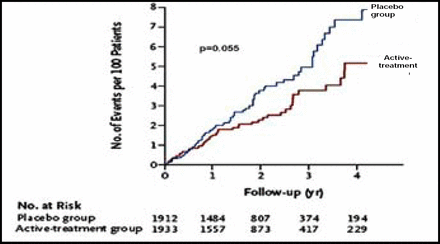
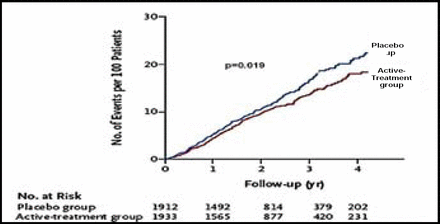
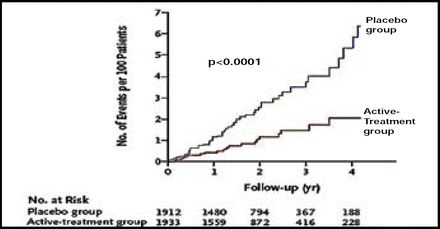
After 2 years of follow-up, significantly more patients in the active treatment group (48.0%) reached the target blood pressure level of 150/80 mm Hg compared with those in the placebo group (19.9%; p<0.001). The rate of fatal or nonfatal stroke was reduced by 30% (Figure 1) with active treatment (12.4% vs 17.7%, HR 0.70 [0.49, 1.01]; p=0.06). With respect to the secondary endpoints, active treatment was associated with a 39% reduction in the rate of death from stroke (p=0.055), a 21% reduction in the rate of all-cause mortality (p=0.019; Figure 2), a 23% reduction in the rate of death from cardiovascular causes (p=0.06), and a 64% reduction in the rate of heart failure (p<0.0001; Figure 3). The benefits of treatment were apparent within the first year.
Stroke Reduction.
Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Total Mortality.
Heart Failure Reduction.
Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Significantly fewer serious adverse events were reported by subjects who received active treatment (n=358) versus those who received placebo (n=448; p=0.001). Only 5 of these events were considered to be related to study treatment (3 in the placebo group and 2 in the active treatment group).
“The results of this study will have important implications for the generation of future guidelines and mean that very elderly individuals with sustained blood pressures of 160 mm Hg or more should now be appropriately assessed and treated in accordance with the new findings,” said Dr. Beckett, adding that, “It's never too late to initiate antihypertensive medications.”
- © 2008 MD Conference Express