Summary
This article discusses updates in stroke recovery therapies, including an overview of the current state of Functional Electrical Stimulation, which is the use of electrical stimulation (via a neuroprosthesis) to activate muscles in a specific programmed sequence to allow the functional use of a limb that has been affected by stroke or spinal cord injury. Also discussed are advances in the BrainGate Neural Interface System, and the development of an interactive robot-assisted neuro-rehabilitation system called RUPERT (Robotic Upper Extremity Repetitive Trainer).
- extrapyramidal & movement disorders
- cerebrovascular disease
John Chae, MD, Case Western Reserve University, Cleveland, OH, presented an overview of the current state of Functional Electrical Stimulation (FES), which is the use of electrical stimulation (via a neuroprosthesis) to activate muscles in a specific programmed sequence to allow the functional use of a limb that has been affected by stroke or spinal cord injury. He also discussed how the use of FES facilitates motor relearning (the ability to reacquire motor skills following brain injury). Dr. Chae reminded the group that motor relearning is activity-dependent and that to achieve maximum therapeutic benefit, the activity in question must be highly repetitive, but more importantly, novel (ie, requires the acquisition of new skills) and functionally relevant.
Most FES neuroprostheses for the stroke population have been devised for lower limb applications. They are effective in enhancing the walking speed of stroke survivors compared with no device [Kottink et al. Artificial Organs 2004]. Commercially available devices provide surface stimulation to the peroneal nerve. Timing is controlled by either a heel switch or tilt sensor. These devices provide balanced ankle dorsiflexion during the swing phase of gait. Surface stimulation has several limitations, however, including discomfort, difficulty with proper placement of electrodes, and inconsistency of response. Several groups in Europe and North America are developing implantable devices to offset these limitations.
The development of upper limb neuroprostheses for stroke survivors is not quite as advanced and must await additional technical and scientific developments. Nevertheless, simple devices may provide some degree of motor relearning. With electromyography (EMG)-triggered electrical stimulation (ES), the stroke survivor initiates an EMG activity by attempting to open the hand. The EMG activity is detected and processed. If the signal exceeds a pre-set threshold, the stimulator provides the ES for full hand opening. Due to the increased cognitive content, several reviews suggest that EMG-triggered ES may be more effective than cyclic ES without an EMG trigger. [de Kroon et al. Clin Rehabil 2002; de Kroon et al. J Rehabil Med 2005].
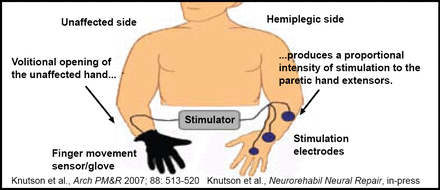
Dr. Chae is currently in the midst of a multicenter clinical trial that is comparing cyclic, EMG-triggered, and sensory ES for upper limb recovery, as well as a single-site clinical trial of surface peroneal nerve stimulation for lower limb recovery. The Cleveland FES Center is also currently conducting a pilot study that is testing a sensor glove (Figure 1) that allows stroke survivors to open and close their affected hand with an FES device that is controlled by the intact hand [Knutson et al. Arch Phys Med Rehabil 2007]. This device allows the stroke survivor to perform repetitive tasks that are both novel and functionally relevant, and may provide the highest level of motor relearning.
Contralaterally Controlled Functional Electrical Stimulation Therapy.
Since the discovery by John Donoghue and colleagues [Maynard et al. J Neurosci 1999] that recordings from groups of neurons in the primary cortex, compared with single neuron firing, provide more information about motor behavior, significant advances have been made using neuronal arrays to allow stroke-impaired individuals to control a computer with thought. Leigh R Hochberg, MD, PhD, Massachusetts General Hospital, Boston, MA, and Rehabilitation R&D Service, Providence, RI, spoke to the audience about the recent research advances that have used the BrainGate Neural Interface System. This brain-machine interface uses platform technology to sense, transmit, analyze, and apply the signals from an array of neurons to control either the movement of a computer cursor or other external devices by thought alone.
The 100-microelectrode silicon array, about the size of baby aspirin, is implanted on the surface of the motor cortex that is responsible for limb movement. The signals are transmitted via cables to a set of computers, where they are analyzed and decoded in real time into either the movement of a computer cursor or the control of other external devices such as a telephone, a television, or lights. Although they are only in early clinical testing at this stage (Hochberg et al. Nature 2006), these neurotechnology advances suggest that it may be possible to restore communication, mobility, and independence for patients with paralysis.
Jiping He, PhD, Arizona State University, Tempe, AZ, presented his work on the development of an interactive robot-assisted neuro-rehabilitation system called RUPERT (Robotic Upper Extremity Repetitive Trainer). RUPERT was developed as a low-cost, interactive, easy-to-operate exoskeletal device for arm function rehabilitation both in the clinic and at home. It has five degrees of freedom (shoulder flexion, humeral rotation, elbow extension, forearm supination, and wrist extension, Figure 2) to allow for training and practice of many fundamental arm activities of daily living. It is powered by compressed air and driven by pneumatic muscles for light weight and safety. It is a mobile device, designed to provide alternative therapy choices, that a patient wears at home to continue repetitive therapy on arm functions and enhance physical and cognitive performance. It was designed to use the patient's existing function to an advantage, to provide assistance only as needed when detecting the tendency of stoppage or slowdown, and facilitate, rather than dominate, function during repetitive therapy sessions.
Robotic Upper Extremity Repetitive Trainer.
Control is adaptive, based on the patient's ability and intent to perform a specific task that is evaluated and detected by sensors on the device. The principle of this control design is to require and encourage the patient's active participation in initiating and completing a task rather than perpetuate his reliance on the device to perform the task, while providing quantitative feedback as to performance and result both instantaneously and chronologically. It allows for repetitive therapy on most fundamental functional tasks and provides a therapist options to specify and modify the task complexity based on evaluation of performance and improvement.
In the future, Dr. He hopes to adapt RUPERT for control of more complex tasks, integrate RUPERT and IME (interactive multimodal environment)-based biofeedback for interactive and entertaining rehabilitation, and expand the degrees of freedom and range of motion of this device. When combined with visual and auditory biofeedback, it will stimulate sensory motor integration, a critical process for neural plasticity and motor function recovery. (Acknowledgment: the work is partially sponsored by NIBIB and NICHD in National Institutes of Health).
Following a stroke, 85% of survivors regain the ability to walk; however, more than 70% of those with strokes that affect the middle cerebral artery experience difficulty reaching for and gripping objects. Jane Burridge, MD, University of Southhampton, UK, presented results from her recent study, which examined the use of implanted microstimulators to restore function to the upper limb.
Based on previous studies showing that triggered ES may be more effective than non-triggered stimulation in facilitating upper extremity motor recovery after stroke [de Kroon JR et al. J Rehabil Med 2005], Dr. Burridge and her colleagues studied the feasibility, safety, and therapeutic effect of implanted microstimulators that are injected into muscle and receive power and digital command data from a single external RF coil. The study enrolled hemiplegic subjects who were >12 months post-stroke and who had impaired upper limb function and normal cognitive ability.
Seven subjects were successfully implanted with 5–7 microstimulators. After a 12-week period of functional exercise using personalized activity programs supported by electrical stimulation, improvement was noted in function (ARAT scores), impairment motor scores (Fugl-Meyer), motor control (Tracking Index), and spasticity (Stretch Index). The largest gains were seen in patients <2 years post-stroke. There were no infections or delayed wound healing. Six of the seven subjects continue to use the system at home. Dr. Burridge looks forward to the next generation of microstimulators and the feasibility of using fewer devices.
- © 2008 MD Conference Express