Summary
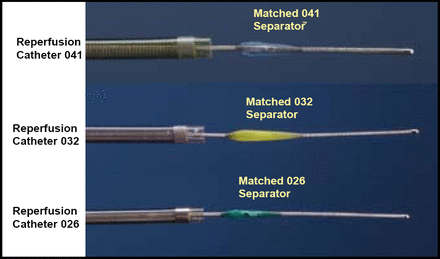
Currently, the only approved treatments for thromboembolus in acute stroke are the thrombolytic agents such as tPA. However, clot-removing drugs have a high risk of bleeding complications, and they are not as effective in patients with severe strokes. The Penumbra System is a novel mechanical device designed to remove stroke-associated occlusions from the large brain vessels and is approved for use in acute stroke. The system comprises an aspirating reperfusion catheter, a separator for clearing, and a thrombus removal ring that is designed to capture calcified, hard clots.
- cerebrovascular disease
- interventional techniques & devices clinical trials
Currently, the only approved treatments for thromboembolus in acute stroke are the thrombolytic agents such as tPA. However, clot-removing drugs have a high risk of bleeding complications, and they are not as effective in patients with severe strokes. The Penumbra System is a novel mechanical device designed to remove stroke-associated occlusions from the large brain vessels and is approved for use in acute stroke. The system comprises an aspirating reperfusion catheter, a separator for clearing, and a thrombus removal ring that is designed to capture calcified, hard clots (Figure 1).
Aspiration System.
Cameron McDougall, MD, Barrow Neurological Institute, Phoenix, AZ, reported results from an international multi-center study that was conducted in 125 stroke patients with occluded vessels. The objective of the trial was to assess the safety and the revascularization effectiveness of this novel clot removal system. Patient inclusion criteria included NIH Stroke Scale (NIHSS) score ∼8, presentation within 8 hours of symptom onset, and a Thrombolysis in Myocardial Infarction (TIMI) score of 0 or I. The primary endpoints were TIMI score II or III and device-related serious adverse events (SAE). Secondary endpoints included ≥4 point improvement on the NIHSS at discharge or modified Rankin Scale (mRS) ≤2 at 30 days, all cause mortality, and incidence of intracranial hemorrhage (ICH).
The median time from symptom onset to procedural start was 4.1 hours; the median time required for revascularization was 45 minutes. Using the Penumbra System, 82% of the treated vessels were revascularized to TIMI II or III, with 41.6% of the patients having a favorable outcome at 30 days. None of the SAEs (3%; 2 perforations; 2 ICHs) was device-related. A total of 35 patients (28%) were found to have ICH at 24 hours, of which 14 (11.2%) were symptomatic (CT evidence of a bleed and a 4-point drop on the NIHSS) and 21 (16.8%) were asymptomatic. At the time of discharge, 58% of the patients had a ≥4 point improvement in NIHSS, and 27% had a ≥10 point improvement in NIHSS or NIHSS 0–1. All cause mortality was 26.4%, and 32.8%, respectively, at 30 and 90 days; 25% of patients had a 90-day mRS of ≤2. Due to the effectiveness of the initial aspiration, direct thrombus extraction was used in only 30 of 125 patients.
The Penumbra System was associated with a low rate of serious procedural complications and had an acceptable rate of ICH and all cause mortality. The trend for a better outcome when vessels were opened was consistently observed across all neurological and functional measures.
The US Food and Drug Administration granted clearance of the Penumbra System for revascularization of intracranial vessels in patients with acute ischemic stroke in December 2007. The retrieval ring was used in a minority of patients, and as a result, it was not felt to have been adequately evaluated and therefore was not approved. No complications or adverse events occurred as result of the use of the retrieval ring.
- © 2008 MD Conference Express