Summary
A number of observational studies have shown that the risk of a poor outcome after acute intracerebral hemorrhage, including early death, is greater among patients who present with higher blood pressure (BP). For every 1–2 mm Hg increase in systolic BP there is approximately a 1% increase in death and dependency. However, the effects associated with early lowering of BP are less clear. This article discusses results from the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial [INTERACT].
- ischemia clinical trials
A number of observational studies have shown that the risk of a poor outcome after acute intracerebral hemorrhage (ICH), including early death, is greater among patients who present with higher blood pressure (BP). For every 1–2 mm Hg increase in systolic BP (SBP) there is approximately a 1% increase in death and dependency. However, the effects associated with early lowering of BP are less clear.
Prof. Craig Anderson, Stroke Medicine and Clinical Neuroscience, Royal Prince Alfred Hospital, Sydney, Australia, presented results from the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT). This was an open-label, pilot phase, randomized controlled trial that enrolled 404 patients from 44 hospitals in Australia, China, and Korea from November 2005 to April 2007. Patients ≥18 years with SBP of 150–220 mm Hg were assigned to receive a treatment strategy of either intensive BP lowering (target SBP 140 mm Hg) based on a stepped protocol of routinely available intravenous agents or the 1999 American Heart Association (AHA) guideline-based BP lowering (target SBP 180 mm Hg within 6 hours of ICH. ICH was confirmed by computerized tomography (CT) scan. The primary outcome was proportional growth in hematoma volume on repeat CT at 24 hours. Clinical outcomes and adverse events were assessed over 90 days.
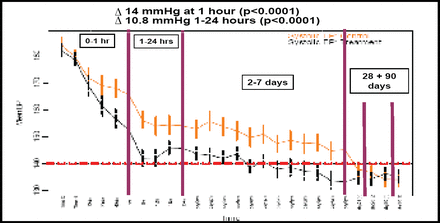
SBP was an average of 14 mm Hg lower (p<0.0001; Figure 1) in the intensive BP lowering group compared with patients in the AHA guideline group at 1 hour post-randomization. Mean proportional hematoma growth was 22.6% lower (95% CI 0.6–44.5%; adjusted p=0.06) in the intensive group compared with the guideline group (36.3 mL vs 13.7 mL), after adjustment for initial hematoma volume and time from ICH to CT. This equated to about 2 mL less blood in the brain associated with early intensive BP lowering (Table 1.) Likewise, ‘substantial’ hematoma growth (ongoing bleeding >33% or 12.5 mL) was 36% (95% CI 0–59%; p=0.05; Table 1) lower in the intensively managed group. There was no evidence of differences in type or frequency of serious adverse events between the two treatment approaches or in clinical outcome at 90 days.
Indices of Hematoma Growth.
Mean (95% CI) Systolic BP Differences.
The results of the INTERACT study show that early intensive blood pressure lowering with careful monitoring is feasible, safe, and well tolerated, and appears to produce a modest attenuation of bleeding and hematoma growth in ICH. Because antihypertensive agents are inexpensive and widely available, widespread adoption of BP lowering could translate into high absolute benefits. A large-scale trial powered to evaluate clinical endpoints is planned to commence later this year.
- © 2008 MD Conference Express