Summary
This article reports the findings of the Brief Infusion of Eptifibatide Following Percutaneous Coronary Intervention [BRIEF-PCI] trial. This trial explored whether the infusion of eptifibatide after uncomplicated percutaneous intervention with stenting could be safely shortened to <2 hours without increasing the risk of ischemic events and with the potential benefit of lowering the risk of major bleeding.
- cardiology clinical trials
- interventional techniques & devices
This trial explored whether the infusion of eptifibatide after uncomplicated percutaneous intervention (PCI) with stenting could be safely shortened to <2 hours without increasing the risk of ischemic events and with the potential benefit of lowering the risk of major bleeding.
“This change in treatment [duration] reduces drug costs up to 37% of the standard regimen and significantly reduces the length of stay and associated costs,” said Anthony Fung, MD, Vancouver General Hospital, Canada, who reported the findings of the Brief Infusion of Eptifibatide Following PCI (BRIEF-PCI) trial.
The BRIEF-PCI trial was designed to compare the standard 18-hour infusion of eptifibatide with a short infusion (<2 hours) after non-emergent PCI. Dr. Fung noted that contemporary PCI involves the use of dual oral antiplatelet therapy, including a loading dose of clopidogrel, and the routine use of coronary stents. The investigators' hypothesis, he said, was that this change in approach to PCI may obviate the need for prolonged infusion of eptifibatide.
The patients in the trial had stable angina, acute coronary syndromes, or recent (within <48 hours) ST-elevation myocardial infarction (STEMI). All patients received intravenous eptifibatide during PCI, and approximately two-thirds of the patients were treated with a loading dose of clopidogrel. After successful PCI, patients were randomly assigned to receive either standard eptifibatide (312 patients) or the short infusion (312 patients). The primary endpoint was ischemic myocardial injury within 24 hours after PCI, as determined by elevated levels of troponin-I and creatinine kinase MB at 6 and 18 hours compared with baseline. The trial was designed as a noninferiority study, with a reference event rate of the primary endpoint of 50% and an upper margin set at 10%. The study was powered at 80%, with a one-sided alpha of 0.05.
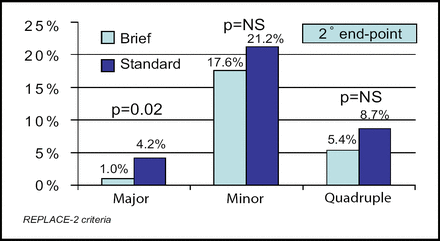
Dr. Fung reported that ischemic myocardial injury occurred in 30.1% of the patients who received the short infusion and in 28.3% of those who received the standard infusion (noninferiority margin, 1.8% [95% CI, 7.8%]; p<0.012 for noninferiority). There was also no difference between the two groups in the rate of the individual secondary endpoints of death, nonfatal MI, urgent target vessel revascularization, or a composite of these events. The incidence of minor bleeding did not differ between the two groups (17.6% vs 21.2%), but major bleeding occurred less frequently among patients who received the short infusion (1.0% vs 4.2%; p=0.02; Figure 1).
Bleeding and Quadruple Endpoints.
“Many institutions have shortened the infusion of eptifibatide already,” noted Dr. Fung. “The findings of our trial confirm that this change is safe and has the benefit of lower rates of major bleeding.” Because the overall sample size was relatively modest (624 patients) and the trial was designed as a non-inferiority comparison with a 10% absolute difference boundary, larger confirmatory trials are needed to validate these results.
- © 2007 MD Conference Express