Summary
The safety of drug-eluting stents (DES) has been a matter of controversy with a recent US Federal Drug Administration Advisory Panel meeting convened. This article presents the results of the Massachusetts Stent [MASS Stent] Trial. This study reviewed medical records of all patients who underwent percutaneous coronary intervention (PCI) with stents from April 1, 2003 through September 30, 2004 at non-government hospitals in Massachusetts, USA. The goal of the study was to determine long-term patient outcomes by stent type in a population representative of current United States medical practice.
- interventional techniques & devices clinical trials
The safety of drug-eluting stents (DES) has been a matter of controversy with a recent FDA Advisory Panel meeting convened. Laura Mauri, MD, MSc, Harvard Medical School and Brigham and Women's Hospital, Boston, MA, presented the results of the Massachusetts Stent (MASS Stent) Trial sponsored by the Massachusetts Department of Public Health. This study reviewed medical records of all patients who underwent percutaneous coronary intervention (PCI) with stents from April 1, 2003 through September 30, 2004 at non-government hospitals in Massachusetts. The time period included the introduction of DES to the market, and patients were followed for 24 months following stent placement. The goal of the study was to determine long-term patient outcomes by stent type in a population representative of current United States medical practice.
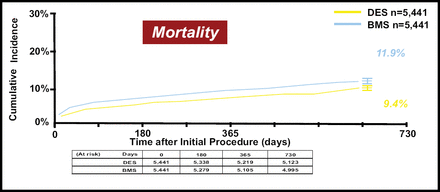
A propensity score-matched analysis was performed using a logistic regression model created from 63 variables. The primary outcome measure was the matched risk differences between the two groups in mortality, myocardial infarction (MI), and revascularization at 2 years. A total of 17,726 patients that underwent placement of a bare metal stent (BMS) or DES were identified; patients that received both stent types were excluded. Sixty-five percent (11,516) of patients received DES compared with 35% (6,210) who received BMS. Of the DES, 72% were sirolimus-eluting, and 28% were paclitaxel-eluting stents. A total of 5,441 propensity matched pairs were analyzed for each of the 2-year outcomes. The DES group was significantly better in terms of mortality, 9.4% versus 11.9% (p<0.0001).
“Previous studies had presented differences in early and late hazards of DES compared to BMS. In fact, what we saw were consistent findings across all time periods,” noted Dr. Mauri (Figure 1). Sensitivity analyses were employed in the following two areas to ensure that residual confounding was not present after the propensity match. The first sensitivity analysis included DES time on the market versus BMS over time, and the second analysis looked at a time-point where there would not be an expected difference in mortality between groups (2 days). The sensitivity analyses findings were consistent with the primary endpoint analyses, indicating that the data are robust. “This [study] demonstrated no increase in rates of death or myocardial infarction associated with DES compared with BMS use at 2 years,” concluded Dr. Mauri.
2-Year Mortality in Matched Patients After DES and BMS Treatment.
- © 2007 MD Conference Express