Summary
More than 2 million patients in the United States take warfarin as anticoagulant therapy. The dose of warfarin may vary depending on patient genotypes, which has resulted in changes in the prescribing guidelines for this agent. The effect of pharmacogenetic (PG)-guided dosing, however, has not been established in controlled studies. The Couma-Gen study was a prospective, randomized, double-blind trial of a PG-guided algorithm for warfarin dosing compared with standard dosing in patients starting oral anticoagulation therapy [Anderson JL, Horne BD, Stevens SM et al. Circulation 2007].
- cardiology clinical trials genomics
- thrombotic disorders
More than 2 million patients in the United States take warfarin as anticoagulant therapy. The dose of warfarin may vary depending on patient genotypes, which has resulted in changes in the prescribing guidelines for this agent. The effect of pharmacogenetic (PG)-guided dosing, however, has not been established in controlled studies.
The Couma-Gen study was a prospective, randomized, double-blind trial of a PG-guided algorithm for warfarin dosing compared with standard dosing in patients starting oral anticoagulation therapy [Anderson JL, Horne BD, Stevens SM et al. Circulation 2007]. Jeffrey Anderson, MD, Intermountain Medical Center, Murray UT, presented the results of the study. Patients ≥18 years of age with an indication for anticoagulation therapy and a target international normalization ratio (INR) of 2–3 were eligible for participation. The trial excluded women of child-bearing potential, patients with severe comorbidity or those taking medications that could confound the measurement of the INR. The standard dosing protocol consisted of 10 mg/day for the first 2 days followed by 5 mg/day (35 mg weekly dose), and then modified as indicated from INR results. The PG-guided algorithm was derived from the patient's genotype, age, weight, and gender. Genotyping was performed using buccal swabs obtained from the patients. Results were generated in, approximately 1 hour. Patients were followed for up to 3 months and INRs were obtained on Days 0, 3, 5, 8, 21, 60, and 90 or longer, if necessary. The primary outcome measure was the percentage of INRs out of range (OOR), characterized by a value either <1.8 or >3.2.
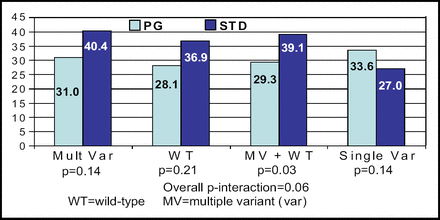
A total of 200 patients were enrolled in the study − 101 in the PG group and 99 in the standard group. The mean follow-up was 46 days, and a mean number of 7.6 INR measurements were made. The groups were well matched, although PG-guided patients were slightly older (63.2 years vs 58.9; p<0.02) and more likely to have hypertension (63.5% vs 47.5%; p<0.02). In terms of genotyping, significantly more patients in the standard group had the VKORC1 1173 CT allele (50.0% vs 35.4%; p<0.05) and any genetic variant (79.6% vs 61.0%; p<0.01). The study did not meet the primary endpoint, as the PG-guided group had 30.7% INRs OOR as compared with 33.1% INRs OOR in the standard therapy group (p=0.47). The PG algorithm, however, did result in significantly fewer INRs per patient and fewer dose changes per patient (Table 1). PG guidance appeared beneficial in wild-type patients and those with multiple variant alleles (Figure 1). “These promising subset analyses will require validation, but for the moment we must consider the clinical benefit of PG-guided warfarin dose initiation to remain unproven,” said Dr. Anderson.
Secondary Endpoints.
Primary Endpoint Subset Analysis: %OOR INR by Variant Status.
In conclusion, Dr. Anderson stressed that larger trials are needed and are now being planned with a National Institutes of Health trial involving 2,000 patients to begin next year.
- © 2007 MD Conference Express