Summary
The final results of the ILLUMINATE trial comparing the cholesteryl ester transfer protein (CETP) inhibitor torcetrapib plus atorvastatin with atorvastatin alone in patients at high risk for cardiovascular disease showed that the rates of major cardiovascular events and all-cause mortality were higher with the addition of torcetrapib. The study had been prematurely terminated in December 2006 because of a significant excess of deaths and cardiovascular events in the group randomized to torcetrapib plus atorvastatin.
- lipid disorders clinical trials
The final results of the ILLUMINATE trial comparing the cholesteryl ester transfer protein (CETP) inhibitor torcetrapib plus atorvastatin with atorvastatin alone in patients at high risk for cardiovascular disease showed that the rates of major cardiovascular events and all-cause mortality were higher with the addition of torcetrapib. The study had been prematurely terminated in December 2006 because of a significant excess of deaths and cardiovascular events in the group randomized to torcetrapib plus atorvastatin.
ILLUMINATE included 15,067 patients at high risk for cardiovascular disease. The study was preceded by a run-in period of 4–10 weeks of treatment with atorvastatin and lifestyle interventions to achieve a low-density lipoprotein (LDL) level of <100 mg/dL. Patients were then randomly assigned to either torcetrapib plus atorvastatin (7,533 patients) or matching placebo plus atorvastatin (7,534 patients).
Philip J. Barter, MD, PhD, The Heart Research Institute, Sydney, Australia, explained that torcetrapib is a CETP inhibitor that has been shown to increase high-density lipoproteins (HDLs) in humans and to protect against atherosclerosis in rabbits. The study hypothesis was that torcetrapib would increase HDL and thus protect against cardiovascular disease.
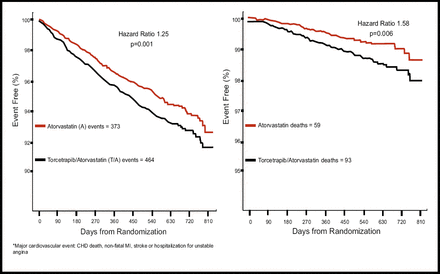
Dr. Barter noted that the HDL and LDL levels in the study indicated that torcetrapib performed as predicted: compared with atorvastatin alone, at 12 months, torcetrapib and atorvastatin increased HDL by 72% (vs 1.8% for atorvastatin alone; p<0.001) and decreased LDL by 25% (vs an increase of 3.0% for atorvastatin alone; p<0.001). However, the drug was associated with a significantly higher number of major cardiovascular events (464 vs 373; p=0.001) and a significantly higher number of deaths (93 vs 59; p=0.006; Figures 1A and 1B). Of note among the deaths, said Dr. Barter, were more deaths in the combination arm related to infection (9 vs 0), cancer (24 vs 14), and stroke (6 vs 0).
Kaplan-Meier Curves for Death from Any Cause and for the Primary Composite Outcome.
Torcetrapib was associated with several off-target pharmacologic effects unrelated to CETP inhibition, said Dr. Barter, such as significant increases in blood pressure, significant changes in serum electrolyte levels, and significant increases in the serum aldosterone level. The higher blood pressure associated with torcetrapib was thought to be related to the greater morbidity and mortality, but post hoc analysis indicated that this was unlikely to be the only explanation, as a greater increase in systolic blood pressure was associated with a lower rate of cardiovascular events. An increase in systolic pressure of more than 2.5 mm Hg was associated with a 5.9% rate of cardiovascular events, whereas an increase of 2.5 mm Hg or less was associated with a 6.3% increase.
Another interesting finding, said Dr. Barter was that in the torcetrapib group, the rate of cardiovascular events was lower in patients who had an increase in HDL-cholesterol (HDL-C) that was greater than the median. At 1 month, the rate of cardiovascular events was 5.9% among the patients who had an increase in HDL-C of more than 22 mg/dL and was 6.4% among patients who had an increase of 22 mg/dL or less. The hazard ratios for cardiovascular-related death or nonfatal myocardial infarction were lower for HDL-C levels that were greater than 60 mg/dL than for a level less than 60 mg/dL. The lowest hazard ratio (0.43; p<0.05) was associated with an HDL-C of more than 93 mg/DL at 3 months.
Dr. Barter emphasized that these post hoc observations are only suggestive and do not rule out HDL dysfunctionality nor the possibility that other unknown effects of CETP inhibition may have contributed to a mechanism-related adverse outcome.
“This study neither validates nor invalidates the hypothesis that raising HDL-cholesterol by inhibiting CETP may be cardioprotective,” said Dr. Barter. “The adverse clinical outcome associated with use of torcetrapib may have been the consequence of an off-target pharmacology but the possibility of an adverse effect of CETP inhibition cannot be excluded by the results of this randomized trial,” he added.
The study findings have been published: [Barter et al. NEJM 2007;357:2109–2122].
- © 2007 MD Conference Express